 OpenEDC
OpenEDC Electronic Data Capture
What is an Innovative EDC System?
Electronic Data Capture (EDC) systems are used worldwide for clinical trials and academic medical research. But what actually makes an innovative EDC system?

EDC Systems Simplify Clinical Trials
What is Electronic Data Capture (EDC) Software?
An Electronic Data Capture (EDC) system is a specialized software solution designed to electronically capture and manage clinical trial data. Instead of relying on paper-based methods, which are prone to errors and time-consuming, an EDC system allows for the direct entry of study data into a digital platform. This data can be captured in real-time from various locations and stored centrally, significantly improving oversight and control over the progress of a study.
Key features of an EDC system include user-friendliness, real-time data validation, audit trails that ensure data integrity, and comprehensive security protocols to protect sensitive patient information. Requirements for an EDC system include high adaptability to different study designs, compliance with regulatory standards (such as Good Clinical Practice (GCP), General Data Protection Regulation (GDPR), and FDA 21 CFR Part 11), and the ability to integrate various data types.
Why Use Electronic Data Capture Software?
The use of electronic data capture software offers numerous advantages for all stakeholders in a clinical study. For study leaders and sponsors, it means a significant reduction in administrative workload, faster data collection and processing, and improved data quality through automated validations. Researchers and clinical staff benefit from a user-friendly interface that simplifies data entry and monitoring, leading to overall more efficient study management.
For patients and study participants, the use of EDC software ensures enhanced data security, as modern encryption methods are employed. At the same time, the risk of errors and delays is minimized, improving the quality of study results. Regulatory authorities and ethics committees also appreciate the traceability and transparency provided by an EDC system.
What Are the Benefits of OpenEDC?
While almost all EDC systems out there are proprietary and have a clunky feel to it, OpenEDC is based on standards, openly available to everyone, and has an innovative take on user-friendliness.

Redefining User-Friendliness
At OpenEDC, we understand that user-friendliness is a crucial factor for any study. The creation of the database, as well as data entry and management, must be straightforward for all users. Issues with the software can lead to rejection by study personnel and thus result in inefficient processes.
For this reason, we designed OpenEDC from the ground up for user-friendliness. The user interface is fully responsive and usable on smartphones, tablets, and computers. It is particularly clear, friendly, and navigable via keyboard—for both study personnel and study participants through electronic Patient-Reported Outcomes (ePRO).
Immediate Availability with Sandbox Environment
Thanks to modern technology, we offer a local, web-based sandbox environment that is immediately accessible to all users worldwide. At app.openedc.health, you can start creating a study right away or import and analyze existing data in HL7 FHIR or CDISC ODM formats. The sandbox environment allows for the rapid design of electronic Case Report Forms (eCRFs) and easy data exchange—100% locally and offline on your computer.
Efficiency Through Data Standards
Many studies share similarities, yet eCRFs are often recreated, leading to high costs and delayed processes. OpenEDC addresses this problem by relying on standardized formats. Create your eCRF once and reuse it as often as you like. Additionally, you can use freely available libraries, such as the Portal of Medical Data Models, to import thousands of eCRFs easily.
Complete Modularity and Expandability
The OpenEDC platform is fully modular and expandable—thus customizable for any study. If you need specific data entry fields, interfaces, charts, or entire modules, they can be integrated with minimal effort. All of this is done through a unified API and fully versioned. Just reach out to us.
End-to-End Encryption for Identifiable Data
Identifiable patient data, such as a person’s name or date of birth, is among the most sensitive information. Nevertheless, this data must be stored in studies to, for example, assign (Severe) Adverse Events and Informed Consents (eConsent) to a person.
As the first EDC system, OpenEDC offers true end-to-end encryption, where data is encrypted on the user’s devices before being sent to a central server. It remains in this state until another user retrieves it from the server and then locally decrypts it with the corresponding key. This exceeds the requirements of the GDPR while being particularly user-friendly and efficient, as no separate paper or Excel sheets need to be maintained.
Insights into the Software
The form editor of OpenEDC offers an interactive and simple way to define the desired structure of clinical data.
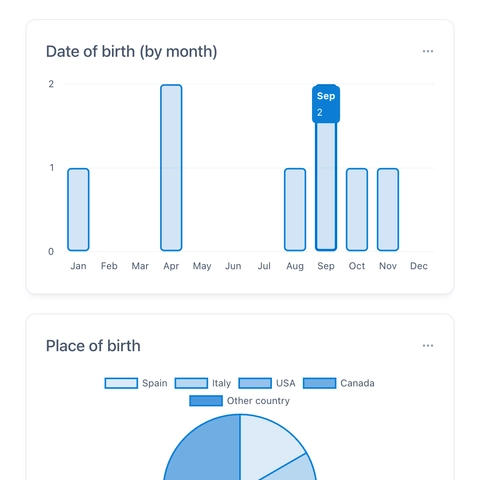
Powerful, highly interactive reports allow for viewing and filtering data in real-time.
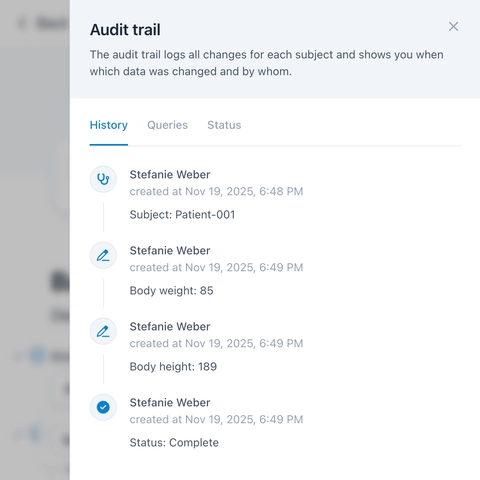
Query Management enables easy task management and ensures high data quality.
Additional Features and Modules
 OpenEDC
OpenEDC Overview of OpenEDC features. Click on a feature to learn more.