OpenEDC Versions 1.4 – 1.6
Scheduled events, a mobile app for study participants, and a collaborative team calendar.

Co-Founder / CEO
To lay the foundation for decentralized clinical trials (DCT) and further simplify collaboration in clinical studies, we are releasing OpenEDC version 1.6 today. In this post, we would like to give you a brief overview of the changes since our last post.
Schedules for Visits and Events
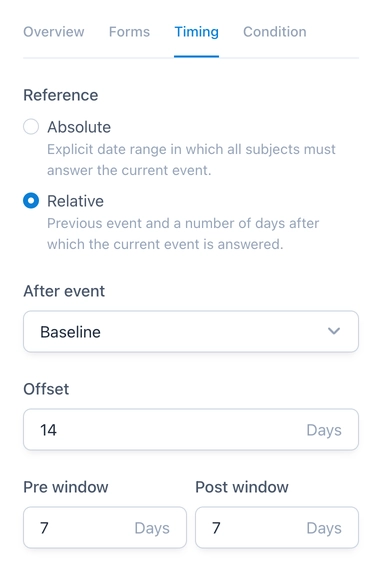
Longitudinal research projects and trials typically consist of multiple visits or events that are often scheduled. OpenEDC now supports the configuration of schedules for such events. Each event can be assigned a time frame during which the event should be filled out by the study staff or answered by a participant. The time frame can either be absolute or relative:
- Absolute time frame: Static date range that is identical for all subjects.
- Relative time frame: Dynamic date range that is calculated based on the response to a preceding event.
To have a complete overview of the study progress, the subject list shows when the next event will take place for each subject. For an even better overview, this is possible in a calendar view, too (see below).
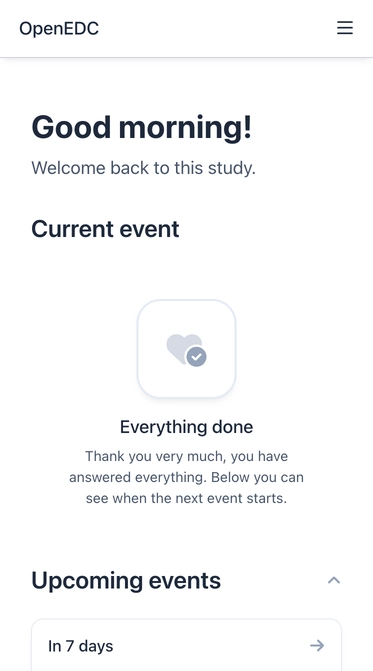
An App for Study Participants with Self-Registration
Based on the schedules for event, a mobile application for study participants is now available. Subjects can sign in and get an overview of all current, upcoming, and past events. This makes it much easier for test subjects to participate in longitudinal studies, puts patients at the center and increases patient compliance in the long term.
To give subjects access to the mobile app, they can now also self-register for a study. This can be configured for a public survey and enables digital patient recruitment in decentralized clinical trials (DCT). After registration, functions such as multi-factor authentication are available for participants, too.
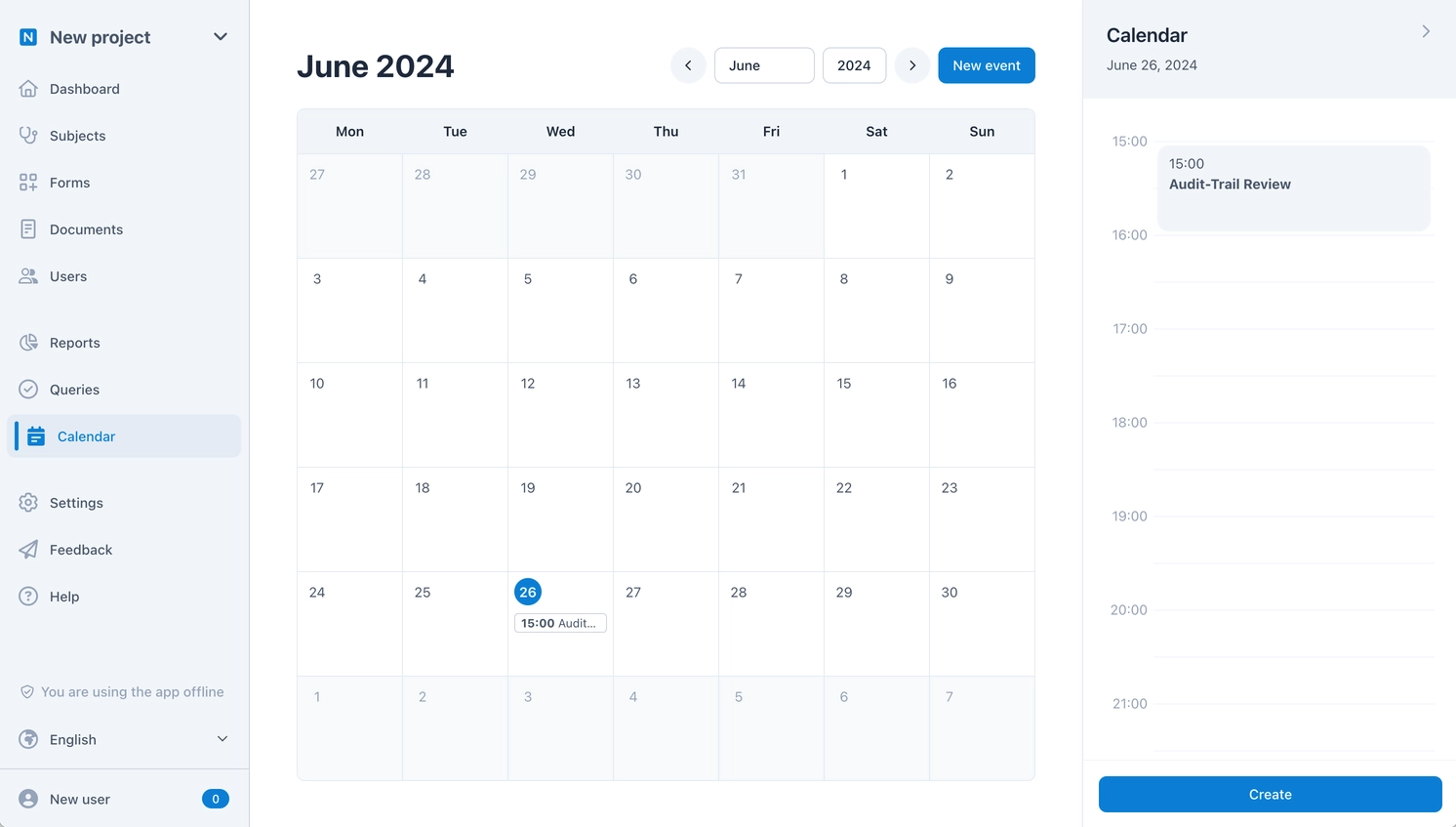
A Collaborative Team Calendar
To fully realize the potential of scheduled events, we have developed a powerful and flexible team calendar. This automatically shows the time frames for all planned visits and for all subjects. For highly efficient appointment management, each time frame can then be planned precisely if needed. The appointments are automatically visible to everyone – without manually sending invitations.
In addition to displaying planned visits, the calendar can also be used for any other appointments. If necessary, such an appointment can also not be shared with the entire team, allowing each team member to organize their own tasks efficiently and centrally – this also applies to task management in query management.
Further Updates
Further changes in the last updates briefly summarized:
Signatures with cryptographic hash function and multi-factor authentication
Digital signatures now contain the cryptographic hash of the values of a form. This allows unequivocal assignment of the signature to a specific point in time of the clinical data. Additionally, a signature can now also be confirmed via multi-factor authentication.
Reason for missing values of an entire form
The reason for missing values can now also be specified for all fields of a form to simplify the documentation of entire missing forms.
Interactive onboarding tour
The web application now includes an interactive onboarding tour that guides users through the application and explains important features. This tour shows how to create a form, add subjects, collect data, and display it in reports.
This concludes this post. Thank you for your interest. If you would like to learn more, please feel free to schedule a non-binding demo.