Changelog
The OpenEDC Health system changelog. Version numbers are assigned according to the Semantic Versioning standard.
v3.6.3 (2026-03-04) Patch
Improvements
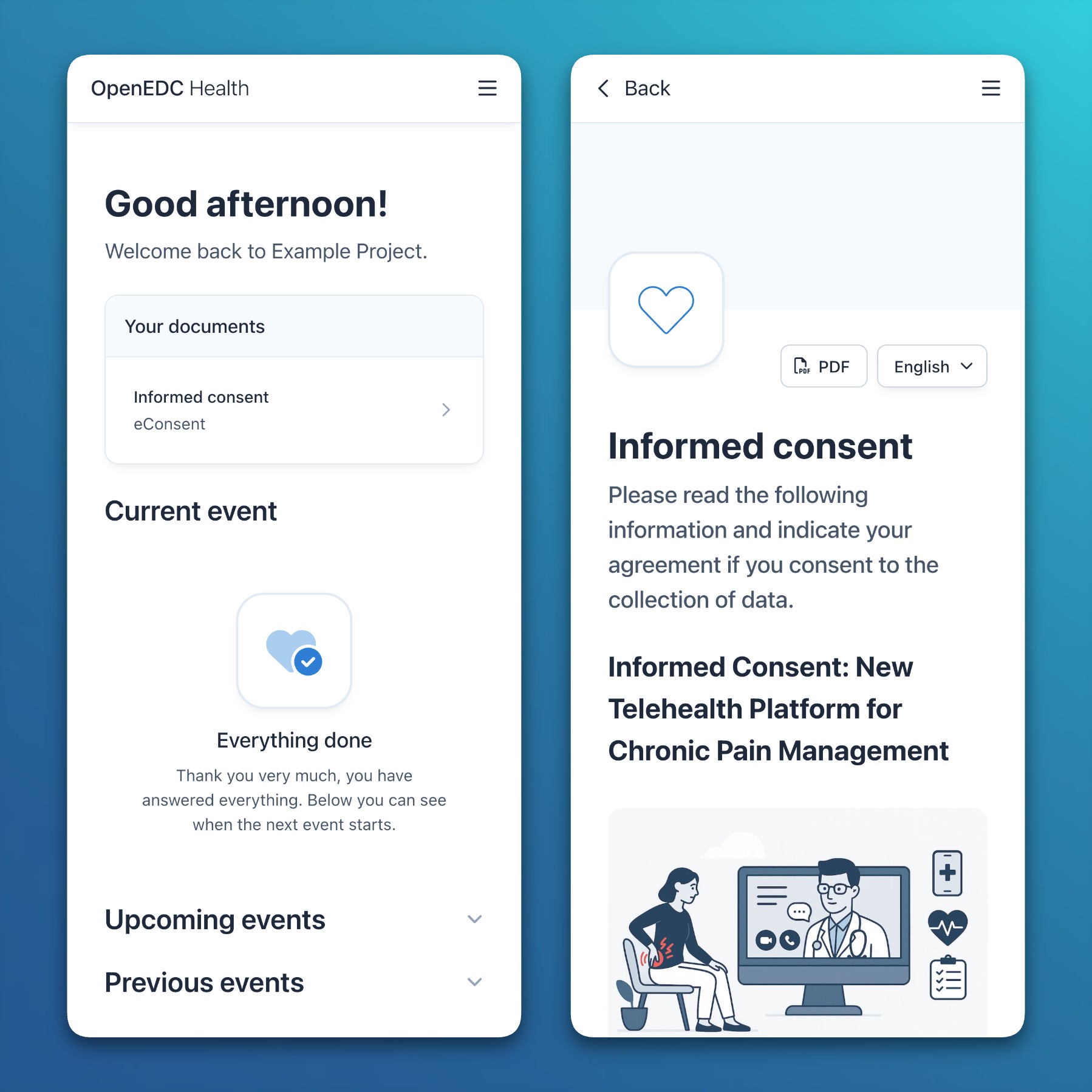
- Informed consent: Study participants can now download signed eConsent documents in PDF format from surveys and the mobile application immediately after completion.
- Participant documents: Important documents (e.g., eConsent or information material) are now displayed and accessible at the top of the mobile participant application.
- Survey settings: Survey settings now include a section with defaults that are automatically applied to new survey links and QR codes, starting with the option to show existing data.
- Informed consent: Withdrawn consent signatures are now visually highlighted with an exclamation mark icon for better clarity.
Bug fixes
- File uploads: Fixed users being unexpectedly logged out due to session timeout or inactivity when uploading large files that took more than 10 minutes.
- File uploads: Fixed file names not being persisted correctly when resuming a previously canceled large file upload.
- Permission system: Fixed the permission to view published documents not being available for the study participant role.
- Participant app: Fixed mandatory fields not being validated during participant data entry, allowing them to be submitted empty.
v3.6.2 (2026-03-02) Patch
Improvements
- Project settings: Added an option to automatically version all forms when moving a project to production.
- Help documents: Added the option to export and print help documents in PDF format.
Bug fixes
- Form versioning: Fixed form status not being linked to the initial form draft, which could lead to incorrect version assignments after publishing.
- Form versioning: Fixed the audit trail to display translated values based on the form version that was active at the time of data entry.
v3.6.1 (2026-02-23) Patch
Improvements
- Authentication: Added Single Sign-On (SSO) support via Keycloak using OpenID Connect (OIDC) for enterprise OpenEDC Health instances.
- Assistant: Added the option to configure custom large language models (LLMs) for the assistant within enterprise OpenEDC Health instances.
- Network: Improved reliability of real-time connections via WebSockets, including an error indication and message for detected connection issues.
- Form editor: In addition to the current event (and event instance), conditions and calculations can now also use the current form (and form instance) as dynamic variables.
- Usability: Improved user interface consistency for footer elements across the application, while also showing more notifications on successful saves.
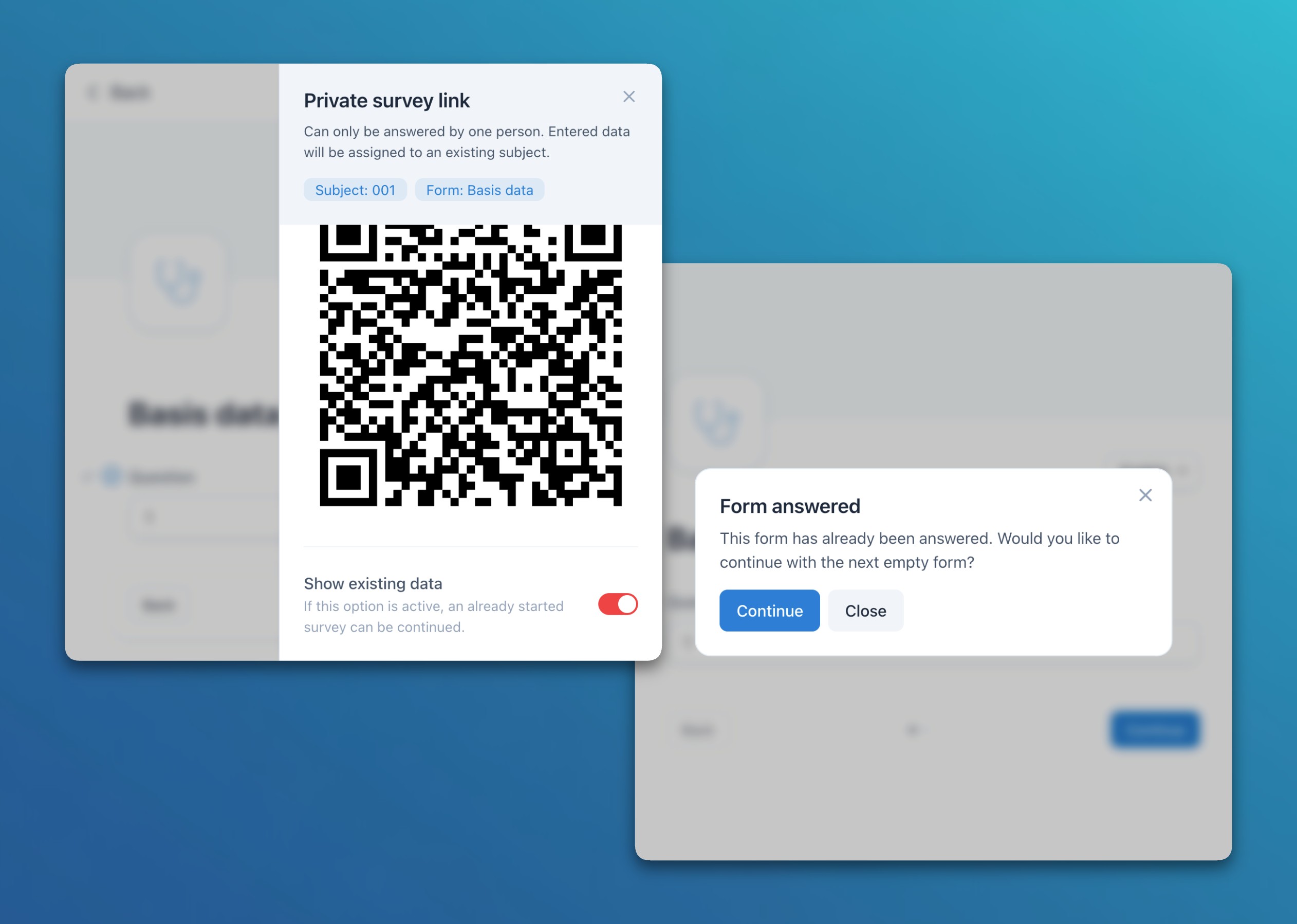
- Surveys: Added an option to show existing form data in individual subject-specific surveys / ePRO links. This is particularly helpful to allow participants to continue an incomplete survey. When opening an answered form, the system also asks if the user wants to go to the first unanswered form.
- Subjects: When a repeating limit is set for events or forms, the subject list and table now display all available instances up to that limit.
- Audit trail: The audit trail now records survey / ePRO link sessions, which allows tracking how often a link or QR code was opened and completed.
- Documents: Added a new link() function to expressions, allowing dynamic clickable links to be created and rendered in documents and descriptive texts.
Bug fixes
- Subjects: Solved an issue where, when both soft and hard validation checks were set for the same item, the soft check could overwrite the hard check depending on their creation order.
v3.6.0 (2026-02-11) Minor
Features
- Automated ePRO invitations via email and SMS
- In addition to push notifications, it’s now possible to configure automated email and SMS messages with embedded ePRO links to study participants according to the defined visit schedule.
- This provides an efficient and accessible alternative to the mobile OpenEDC app and doesn’t require anonymous registrations or app installations.
- SMS messages can be sent internationally and consider site-specific time zones when configured.
- All three notification methods (push, email, and SMS) can be combined, allowing the system to use every available channel to reach study participants.
Improvements
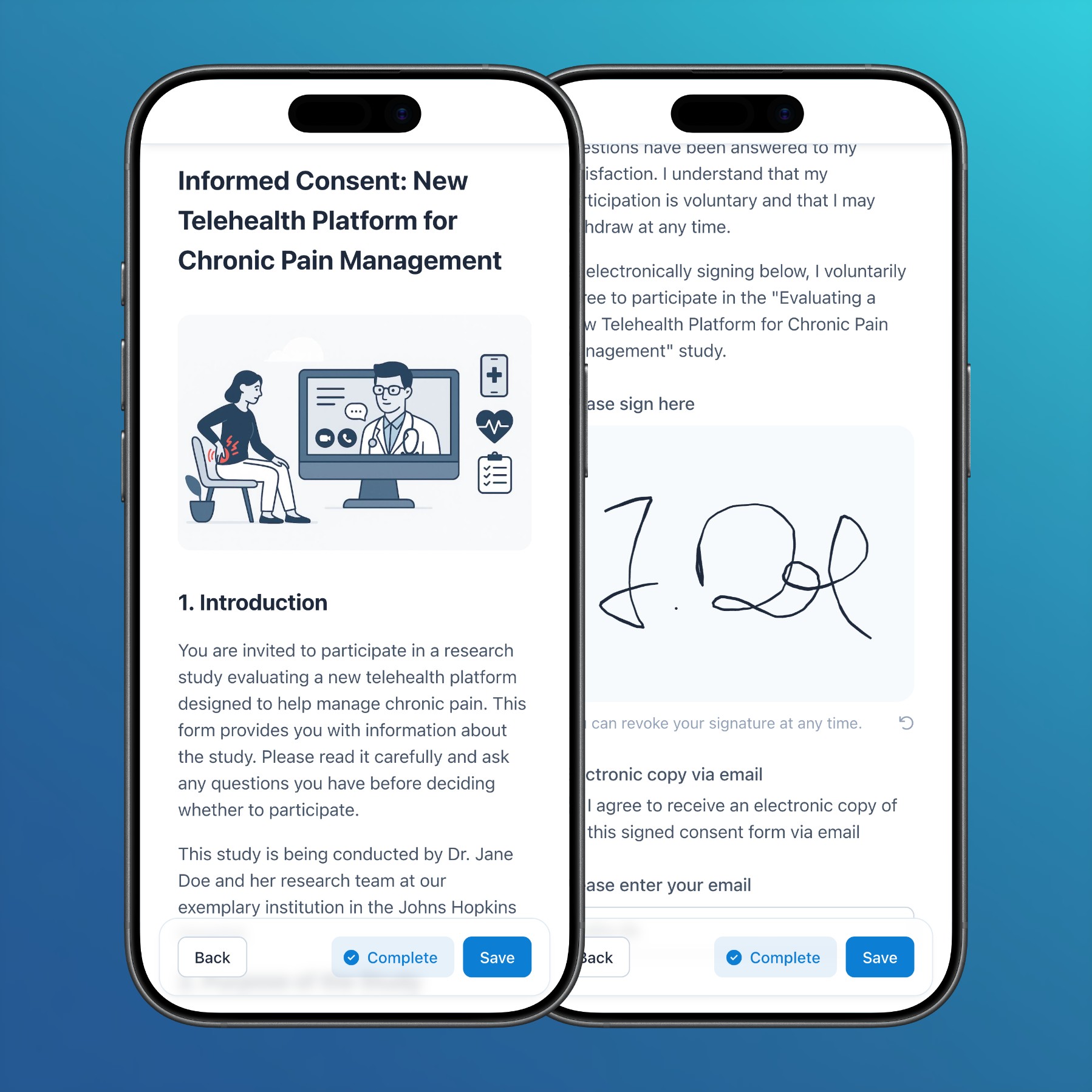
- Subjects: In addition to the web app and participant app, handwritten signatures can now also be captured in surveys and via ePRO links.
- Forms: In PDF exports of forms without clinical data, informed consent fields are now rendered with a gray border to highlight the signature area.
- Reports: In addition to the audit trail within the subjects module, the general audit trail report now also renders change reasons.
- Documents: Besides uploading images, links to images can now be pasted directly into documents to embed them inline.
Bug fixes
- Subjects: Solved an issue that sometimes resulted in change reasons not being rendered correctly in the audit trail.
v3.5.0 (2026-02-02) Minor
Features
- Study and site-specific time zones
- A study-based time zone can now be defined at the project level, and it’s also possible to specify a time zone per site when sites are geographically dispersed.
- Site-specific time zones function as an override, ensuring that sites without a time zone automatically use the one defined at the project level.
- Time zones are primarily used to calculate visit schedules and automatically send push notifications to participants based on their local date and time.
Improvements
- Usability: Added additional data modification confirmation prompts (e.g., when creating form or document drafts) and success notifications (e.g., after changing the project status) throughout the application.
- Forms: For each event, a reference date can now be defined, which is used in visit schedules instead of the event answer date.
- Subjects: Fields in a data matrix now display their value as a tooltip, which is especially useful for disabled inputs where long values may be truncated and not fully visible.
Bug fixes
- Subjects: Solved an issue that caused the data matrix instance buttons to remain visible when the form was disabled.
- Subjects: Solved an issue where the data matrix validated inputs after each typed character, which could result in negative number signs being initially removed when followed by a zero.
v3.4.0 (2026-01-19) Minor
Improvements
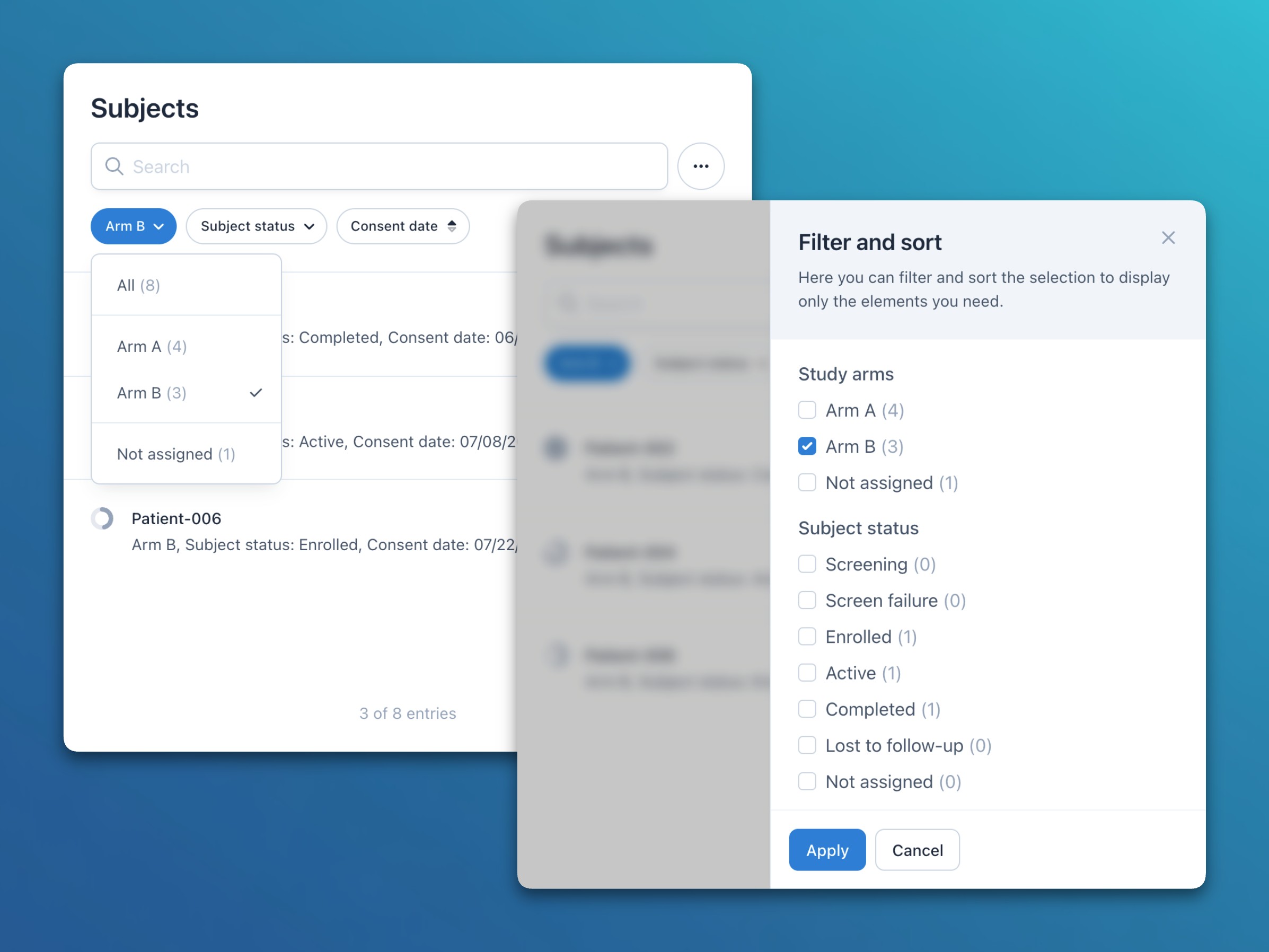
- Usability: For all list filters (e.g., study arms, subject status, or custom fields), the number of elements in each filter group is now displayed in parentheses after the group name.
- Usability: List filters now also include a Not assigned option to find elements that are not assigned to any of the available groups (e.g., subjects without a study arm or status value).
- Forms: In unversioned (i.e., draft) forms, single choice fields can now be transformed to multiple choice fields, while automatically migrating potentially existing data.
- Forms: Study events that are shared across multiple arms (e.g., when different arms have the same visits) are now only displayed once throughout the application.
- Forms: In addition to the list view, assigned study arms are now also displayed in the subject table while form status icons of unassigned arms are omitted.
- Forms: When shallow copying a section, the conditions, calculations, and measurement units of contained items are now also copied.
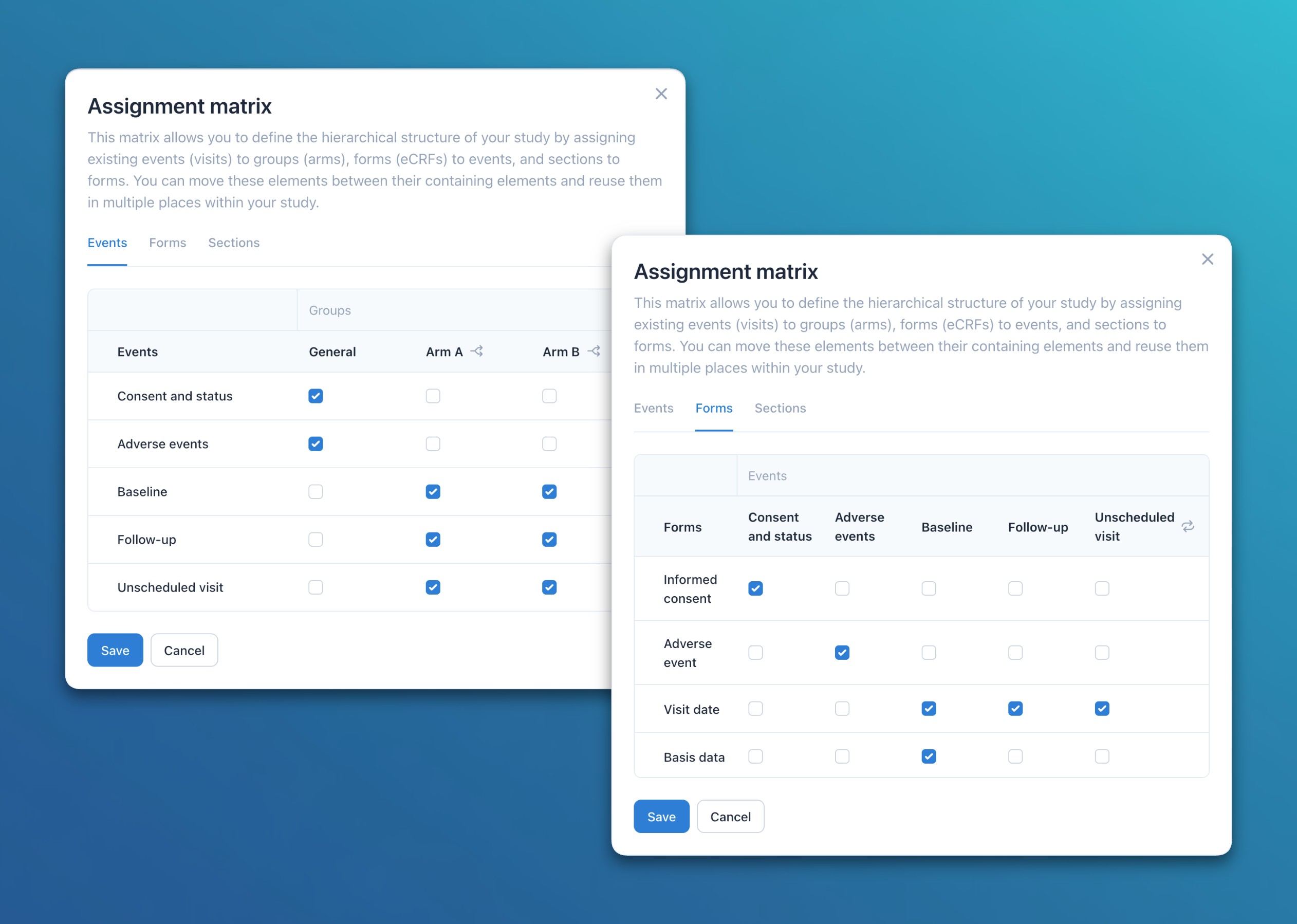
- Forms: Users can now define relationships at three levels with the assignment matrix: Sections to Forms, Forms to Visits, and Visits to Arms. This enables flexible composition and efficient reuse of metadata elements throughout the study.
- Subjects: In addition to repeating event instances, it’s now also possible to send a survey link or QR code for a specific repeating form instance.
- Subjects: For non-repeating data matrices with statically defined rows, it’s now possible to transpose individual rows to normal form sections.
- Subjects: For comprehensive data matrices and Likert scales, the table header now stays at the top while scrolling to remain visible during data entry.
Bug fixes
- Subjects: Solved an issue that resulted in section instances being sorted alphabetically instead of numerically.
v3.3.0 (2026-01-05) Minor
Features
- Copy metadata elements
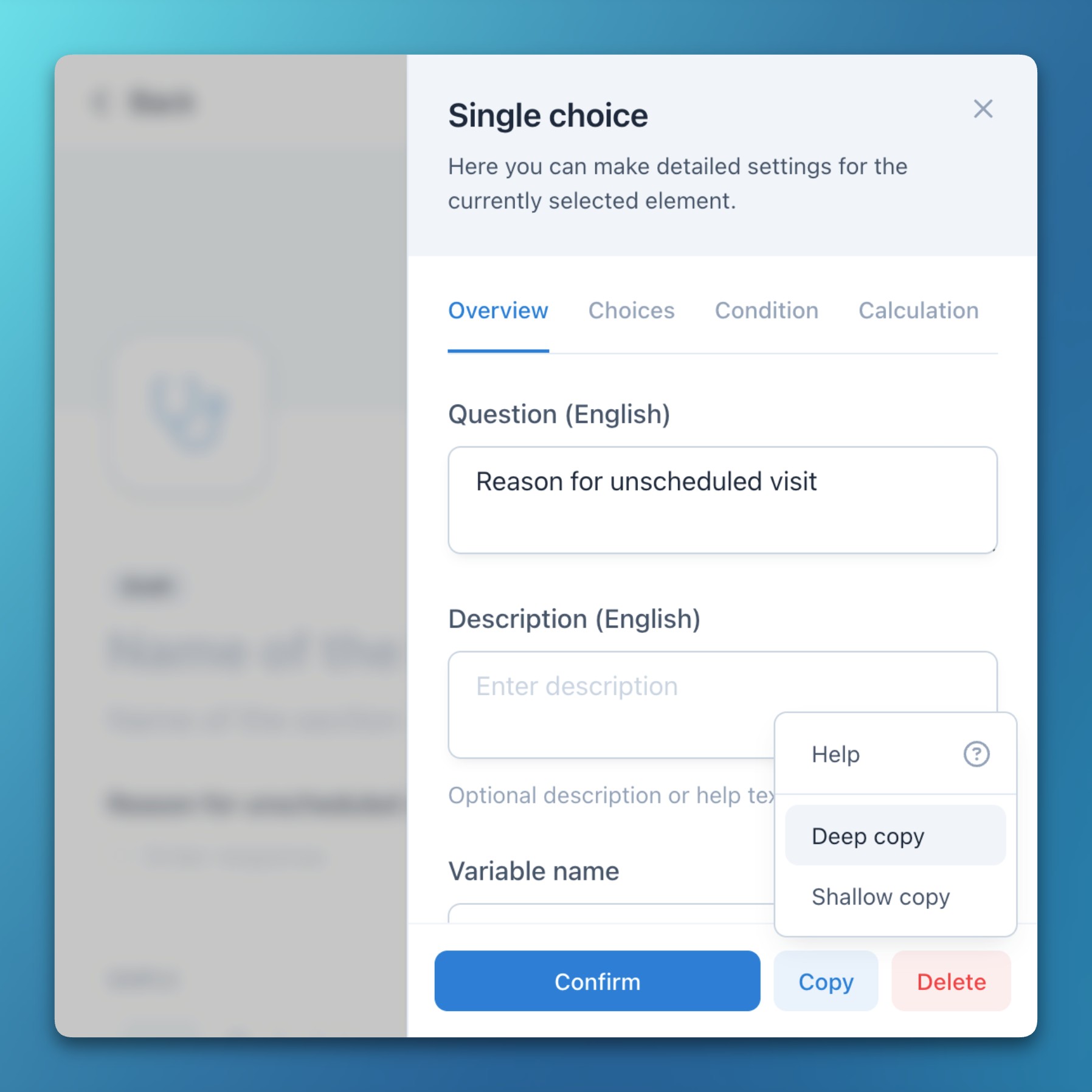
- Study event groups (e.g., arms), events (e.g., visits), forms, sections, item groups (e.g., Likert scales), and items can now be copied using two different methods:
- Deep copy: The entire element and all contained elements (questions, choices, etc.) are fully copied. Everything can then be edited without affecting the original element.
- Shallow copy: Contained elements (questions, choices, etc.) are referenced instead of copied. These therefore automatically adopt subsequent changes or corrections to the original element.
- This feature is particularly useful for creating data matrices, reusing existing forms with minor adaptations, or duplicating study arms.
- Study event groups (e.g., arms), events (e.g., visits), forms, sections, item groups (e.g., Likert scales), and items can now be copied using two different methods:
Improvements
- Forms: The metadata element slideover now includes a dedicated Confirm button. Even though changes are saved automatically by default, this improves interface consistency and also hides the slideover on click.
- Reports: The Tabular overview default report now displays a subject’s location, creation date, form completion status, and answer date.
- Forms: After adding a new field, the form editor now automatically scrolls it into view.
v3.2.0 (2025-12-29) Minor
Features
- Randomization module
- OpenEDC now includes an integrated randomization module with support for variable block sizes and stratification parameters.
- Enabling randomization is a guided four-step process: configuration, preview, review, and confirmation.
- Four additional permissions enable fine-grained control over configuration, review, randomization, and unblinding.
- Atomic database transactions ensure data integrity even when multiple users randomize subjects at the same time.
- After randomizing a subject, the screening ID remains visible in a dedicated information panel when needed.
- All randomization activities and configuration updates are logged in the audit trail.
- Subject key options
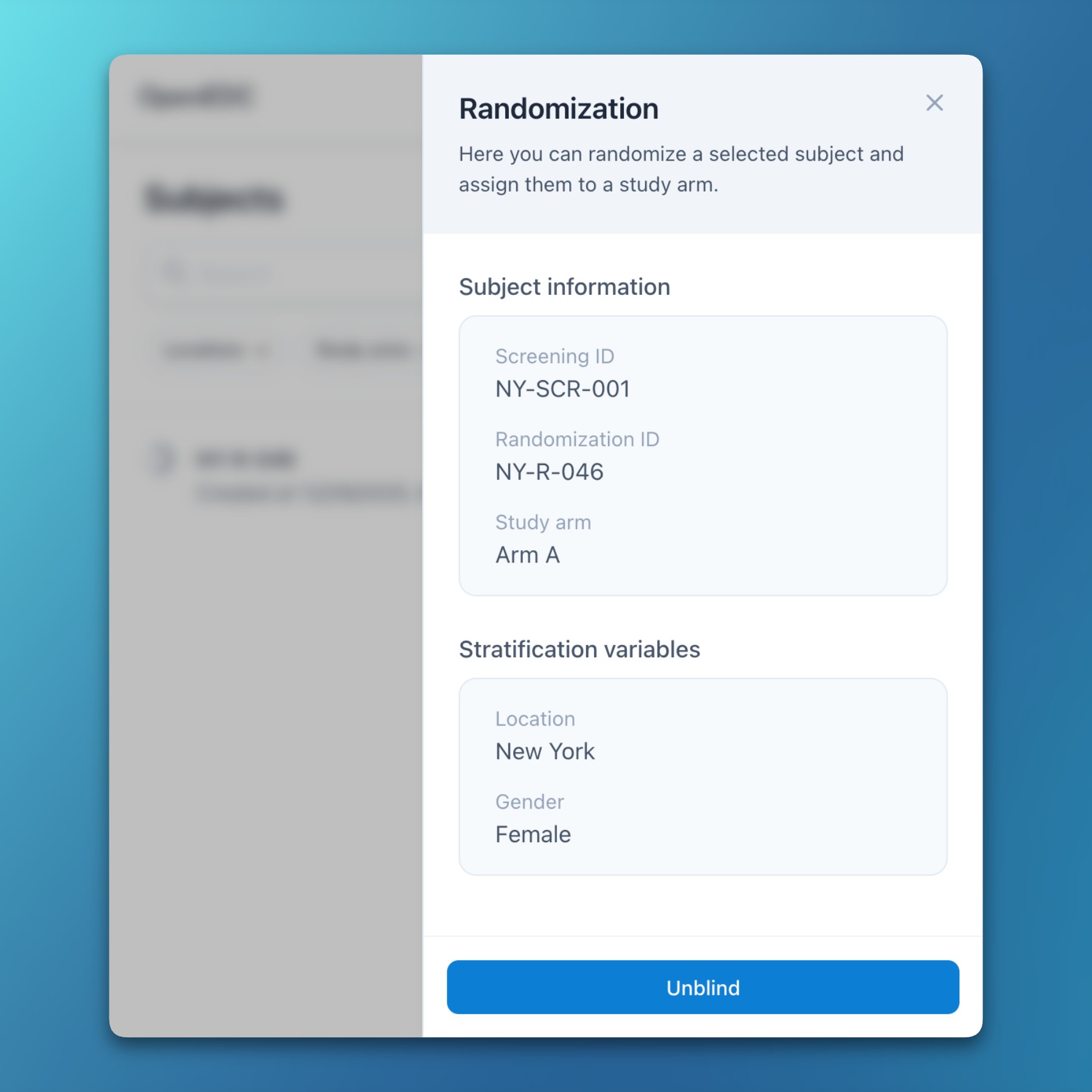
- To support flexible screening and randomization IDs, five new subject key options have been introduced:
- Minimum length: Ensures that subject keys are padded with zeros to a specified length (e.g., 001).
- Randomization/manual prefix: Adds a prefix before the randomized or manually entered key (e.g., R-001).
- Screening/auto-increment prefix: Adds a prefix before the screening or auto-incremented key (e.g., S-001).
- Location ID prefix: Adds the location ID before the key (e.g., NY-R-001 or NY-S-001).
- Location-scoped uniqueness: Requires subject IDs to be unique only within their location instead of globally across the study (e.g., allowing NY-R-001 and SF-R-001). This also applies to auto-incremented keys.
- These options can also be used for subject key customization without the randomization module.
- To support flexible screening and randomization IDs, five new subject key options have been introduced:
Improvements
- Administration: System administrators can now update their enterprise OpenEDC Health instance semi-automatically via the administration module.
- Administration: System starts and updates are now recorded and visible for administrators in a new system audit log table.
Bug fixes
- Network: Solved an issue where auto-incremented subject keys were sometimes not displayed immediately when WebSocket connections were blocked by restrictive firewall settings (e.g., in enterprise environments).
v3.1.1 (2025-12-11) Patch
Improvements
- Administration: The global user and project management dialogs were combined into one administration module for simplified enterprise management.
Bug fixes
- Forms: Solved an issue that resulted in the select field not appearing as an item display option when editing a form layout.
- Importers: Non-printable control characters are now automatically removed from XML files prior to import.
v3.1.0 (2025-12-09) Minor
Improvements
- Users: The server email template now includes a one-line summary for invite and password reset emails.
- Documents: Referenced PDF documents in forms, e.g., for informed consents, are now embedded in PDF/A exports.
- Usability: Unified and extended visual loading indicators across the application, e.g., when creating and updating queries.
- Subjects: Single choice fields now allow answers to be deselected, even when the item is mandatory.
Bug fixes
- Users: Solved a rendering issue that sometimes led to an infinite loading spinner at the top of the user form after inviting a new user to a project.
- Users: Fixed a server-related problem that resulted in system users not being deleted after the last associated project user has been deleted.
- Users: Corrected a permission issue that resulted in an error when trying to import metadata without the Edit project settings permission.
v3.0.0 (2025-11-20) Major
We are happy to announce the next major version of our platform: OpenEDC Health 3.0. This update brings Push and Email Notifications, anonymous Patient Registrations via QR codes, and automated ePRO Invitations with support for repeating events and hourly schedules — along with many quality-of-life improvements.

Features
- Notification system
- Push, email, and in-app notifications can now be enabled per project to automatically inform users and study participants about important events.
- Initially, four notification types are supported:
- Query assignments: Sent to a user when a new query is assigned or an existing query is reassigned to them.
- Subject creation: Sent to all eligible users when a new subject is created, considering permissions and location assignments.
- Schedule reminders: Sent to study participants when a (virtual) visit is due to be answered based on the event schedule (see below).
- System announcements: Sent manually by us to inform all users about system updates. Can now be disabled.
- The preferred notification methods for each notification type can be configured individually for each user.
- Unread notifications are displayed in the new notifications slideover, accessible from the sidebar and dashboard.
- Anonymous registrations
- Study participants or patients can now have anonymous accounts for a study without needing an email address.
- Users can create an authentication QR code for a subject, encoding a One-Time Password (OTP), which can be scanned by the participant to join the study.
- After choosing a password, participants can also install our mobile application on their smartphones or tablets, which allows them to receive push notifications (see above).
- Repeating limits
- For repeating events, forms, and sections, an instance limit can now be configured.
- This limit is visually rendered in the subject list, and after reaching it, no further repeating instances can be created.
- Additionally, the form status aggregation takes the limit into account, automatically calculating a subject’s or event’s completion progress based on the total number of repeating instances.
- Repeating visits in event schedules
- It’s now possible to include repeating visits in the event schedule, while also considering the optionally configured instance limit (see above).
- For example, a Follow-up visit starting 4 weeks (+/- 7 days) after the Baseline will repeat every 4 weeks (until the optional limit is reached).
- Each upcoming visit, including the respective time frame and instance (e.g., 3. Follow-up), is rendered in the subject list and calendar.
- Hourly timing for event schedules
- Event schedules can now be configured at exact points in time, using 15-minute increments.
- For example, a diary can be scheduled to be answered every day at 09:00 in the morning and 18:00 in the evening, for a total of 30 days.
- Individual schedule per participant
- Besides a general hourly timing for scheduled events and push notifications, it is also possible to define a custom schedule for each study participant.
- This can be configured either by the study staff or independently by the participant, if enabled.
Improvements
- Users: Modules are now automatically hidden from the sidebar for users without any read or write permission for those modules. To allow for granular control, new permissions have been introduced for the forms, documents, reports, queries, calendar, and settings modules.
- Subjects: The aggregated form status now automatically switches to Complete when all mandatory fields have been answered, regardless of unanswered optional fields.
- Subjects: In surveys, the pagination indicators at the bottom now consider conditional forms, showing only the number of pages that actually need to be answered.
- Subjects: Surveys via URL or QR code (i.e., without the participant app) now also support repeating study events.
Bug fixes
- Internals: Resolved a local state and rendering issue, where deleted metadata elements (like fields or codelist items) were not visually removed when the containing parent element (like the section or item) was shortly created before.
v2.24.0 (2025-10-13) Minor
Features
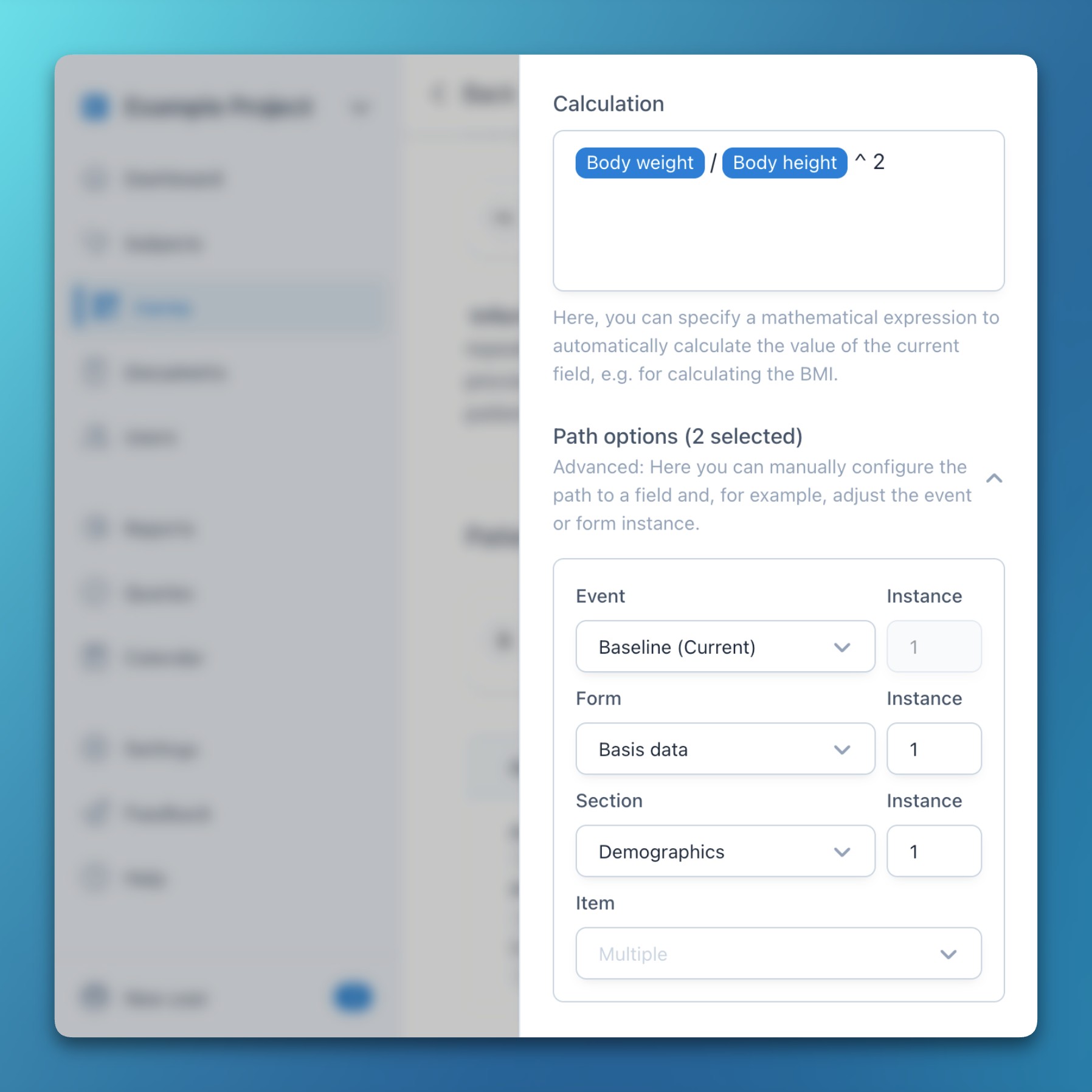
- Path options in expressions
- This new feature for experienced users allows flexible customization of item paths in expressions (conditions and calculations).
- For each item path, the event, form, section, and item can be manually configured – together with the respective repeating instance.
- Multiple items can be selected with CTRL / CMD + Click, allowing the adjustment of multiple references at once (e.g., to extract all data in a formula from one specific repeat instance).
Improvements
- When exporting PDFs of answered forms, conditionally hidden elements (fields and sections) as well as condition texts of shown elements are now excluded.
Bug fixes
- Solved an issue where calculations in descriptive texts resulting in a numeric zero were not correctly rendered in PDF exports.
v2.23.0 (2025-10-03) Minor
Features
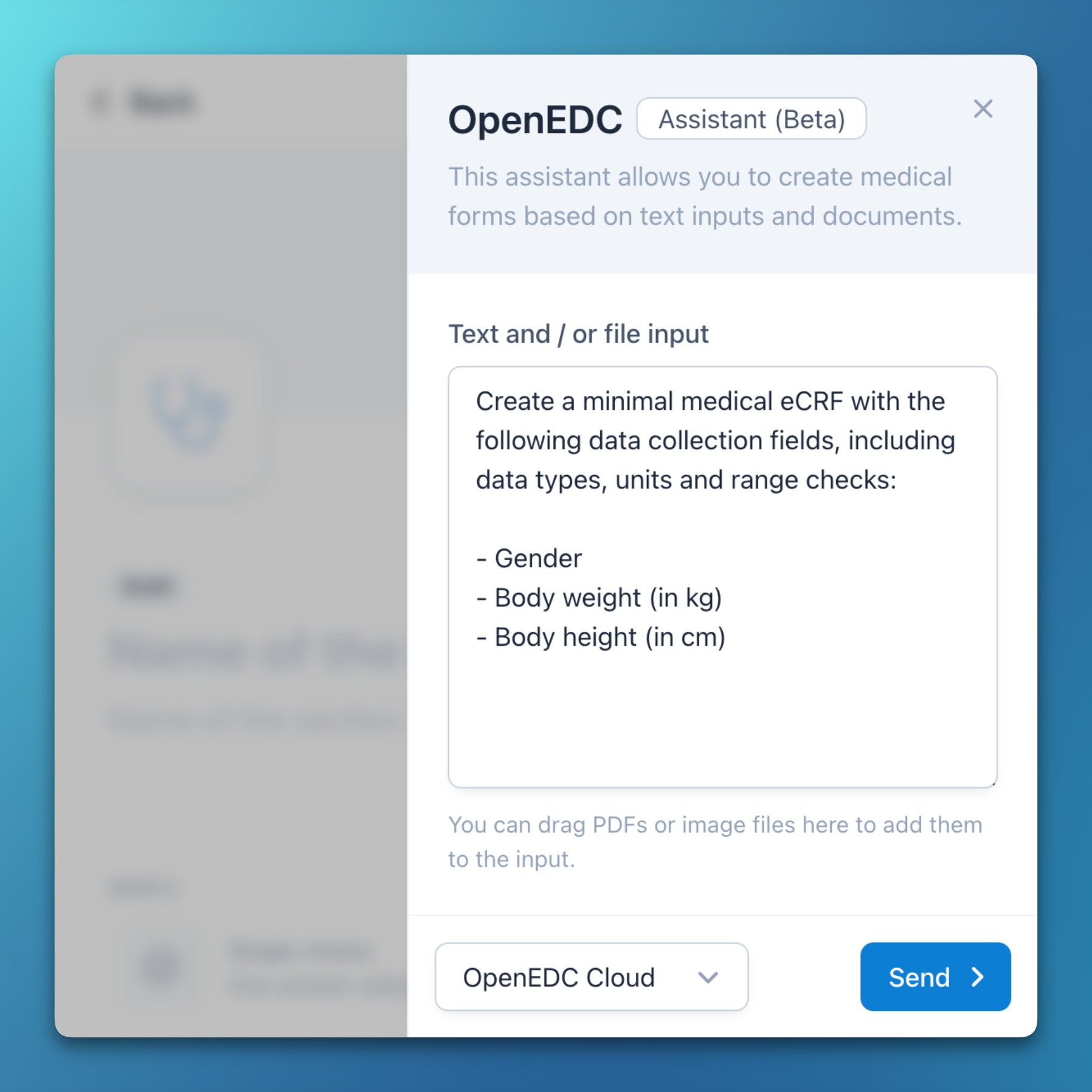
- OpenEDC Assistant (Beta)
- Today, we are excited to announce and release the first beta of the OpenEDC Assistant: A human-in-the-loop and privacy-first AI supporting system that can be used for different purposes across the OpenEDC Health system.
- Every task results in a suggestion which needs to be manually verified and accepted by a human, visually rendered as side-by-side comparison with the current state.
- The assistant can create or edit eCRFs, extract structured medical information, or proofread and translate documents, all based on textual, image, or PDF inputs.
- Our applied AI model is hosted in the Telekom Cloud in Germany. Data is neither used for training, nor logged, analyzed, or stored. Moreover, we provide support for Gemini Nano, a small model that can run entirely offline on modern computers.
v2.22.1 (2025-09-25) Patch
Improvements
- Improved rendering performance by up to 3 times for very large forms containing a few hundred fields.
Bug fixes
- Solved an issue where entering a missing reason for an already persisted but empty field would not immediately render the missing reason above the field.
v2.22.0 (2025-09-23) Minor
Features
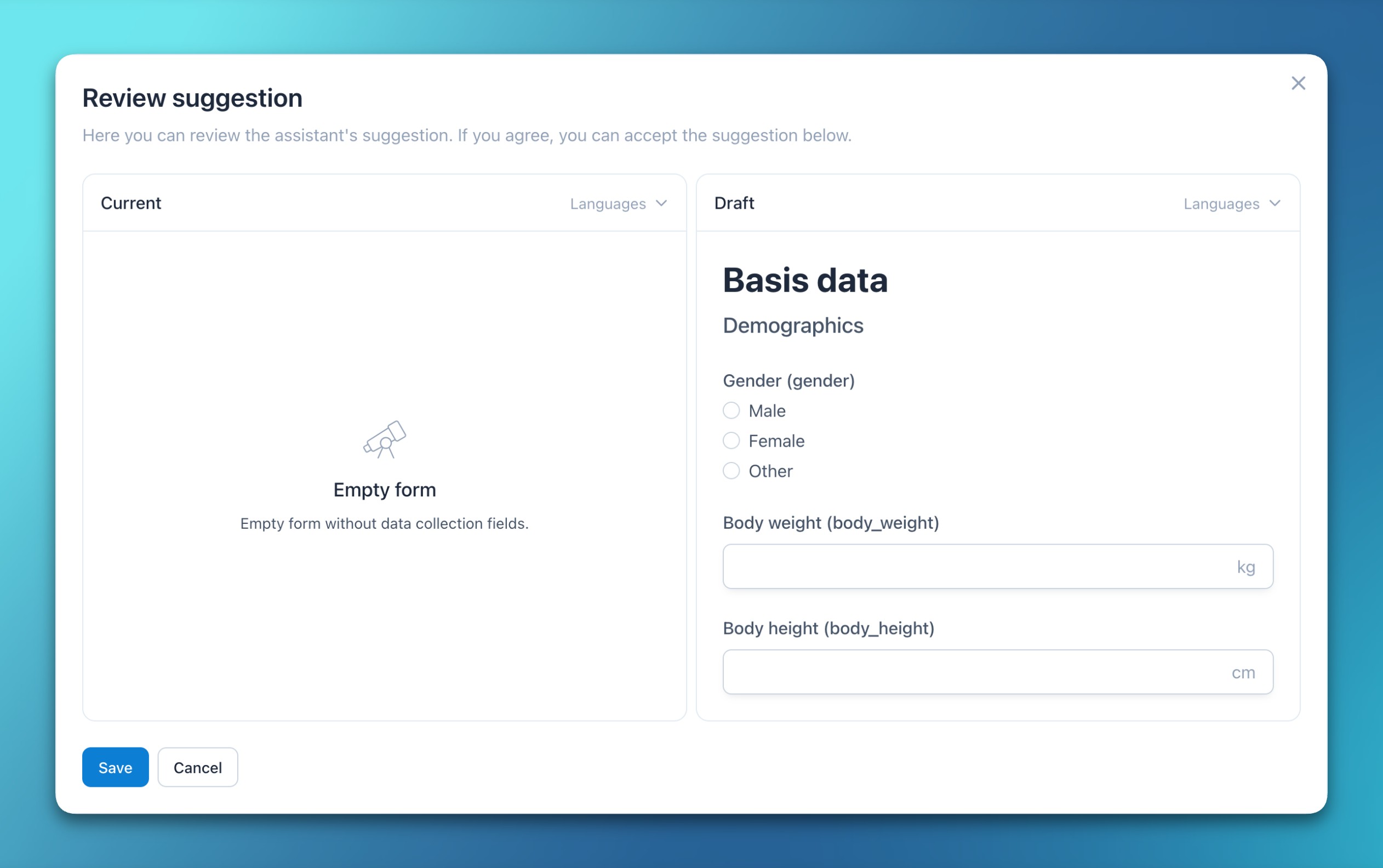
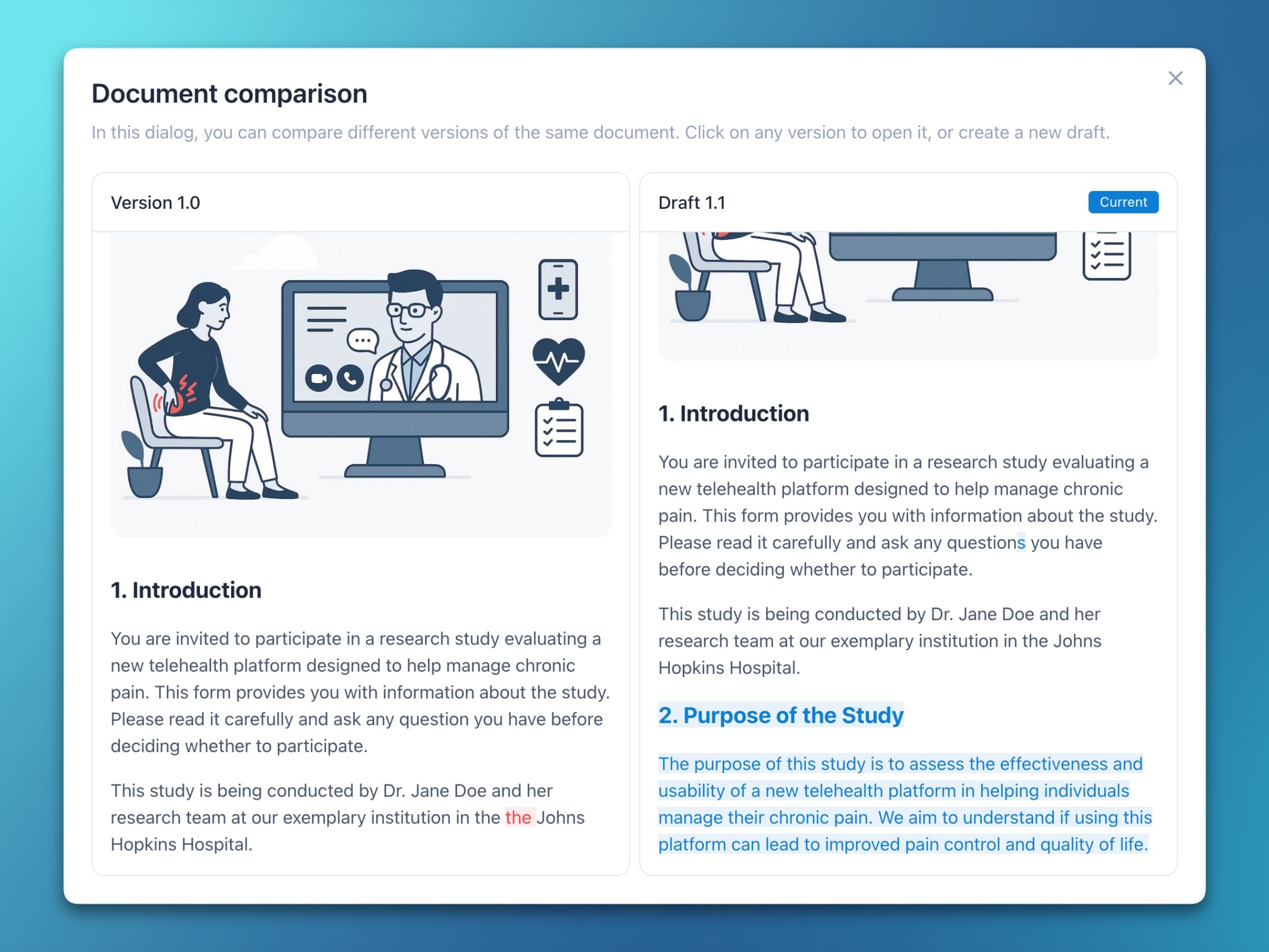
- Form and document comparisons
- Clicking a form or document version identifier now opens a side-by-side view that highlights changes between revisions.
- Selecting one specific version opens it in the respective editor for further processing.
- Deleted and added characters are visually highlighted for improved recognizability.
Improvements
- Significantly reduced the initial loading time of projects with comprehensive metadata.
Bug fixes
- Solved an issue where uploaded files were sometimes not correctly rendered within the form viewer.
- Fixed an error where missing reasons and verification states were not unset when an item became conditionally hidden.
v2.21.0 (2025-09-10) Minor
Features
- Example project
- The system now comes with a comprehensive, multilingual example project to explore many of its features in a realistic environment.
- We added the following events and visits: Informed consent, Adverse events, Baseline, Follow-up, and Unscheduled visit.
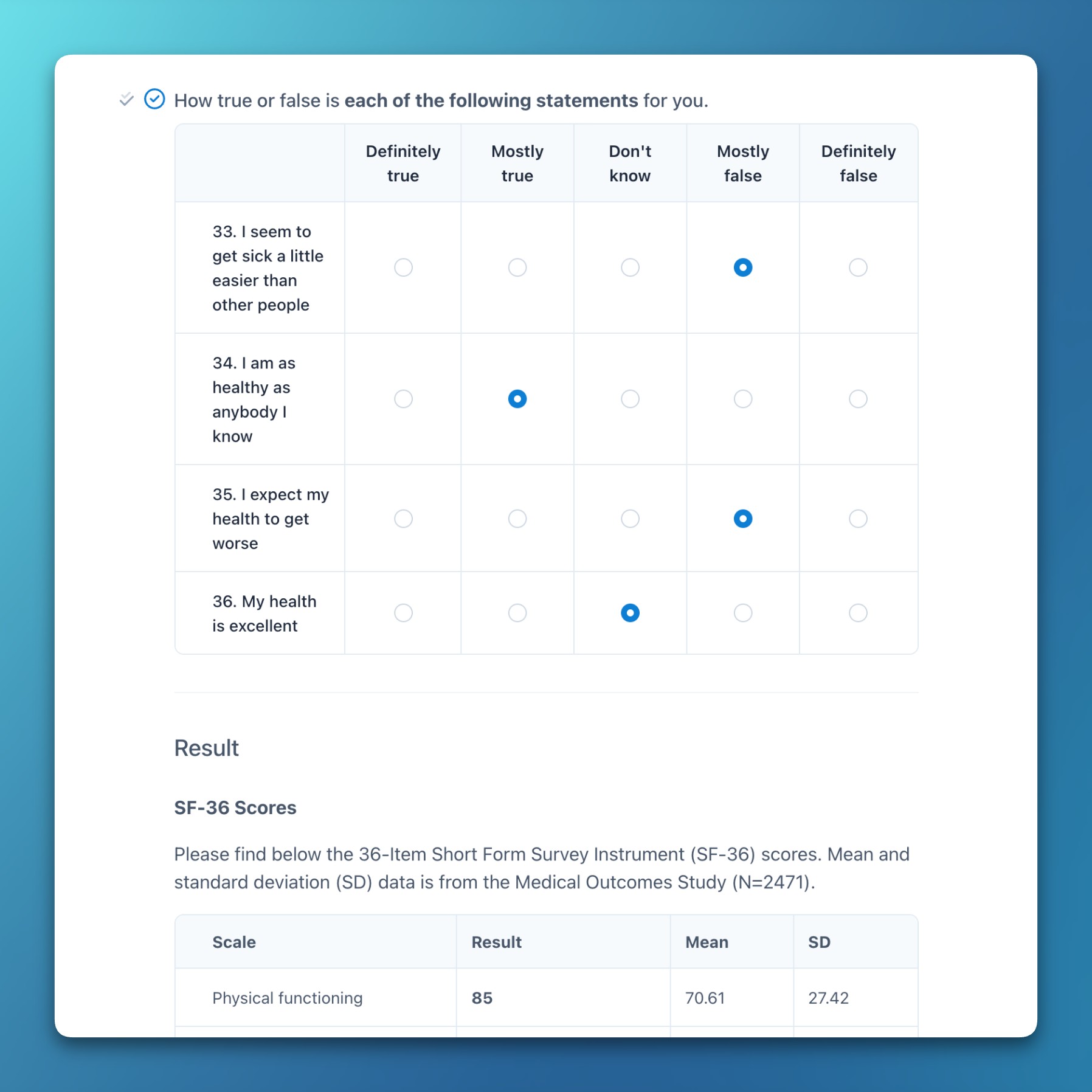
- We integrated the standardized SF-36 and WHOQOL-BREF forms, including score calculation.
- All forms are available in English and German.
- Furthermore, the example project includes complex field validation rules (date not in the future, date not before another date, durations in hh:mm, …), conditional elements (fields, sections, and forms), repeating elements (sections, forms, and visits), data matrices, calculated fields, informed consent documents, and much more.
- To view the example project, visit our sandbox at app.openedc.health and choose Options → Example Project on the welcome screen.
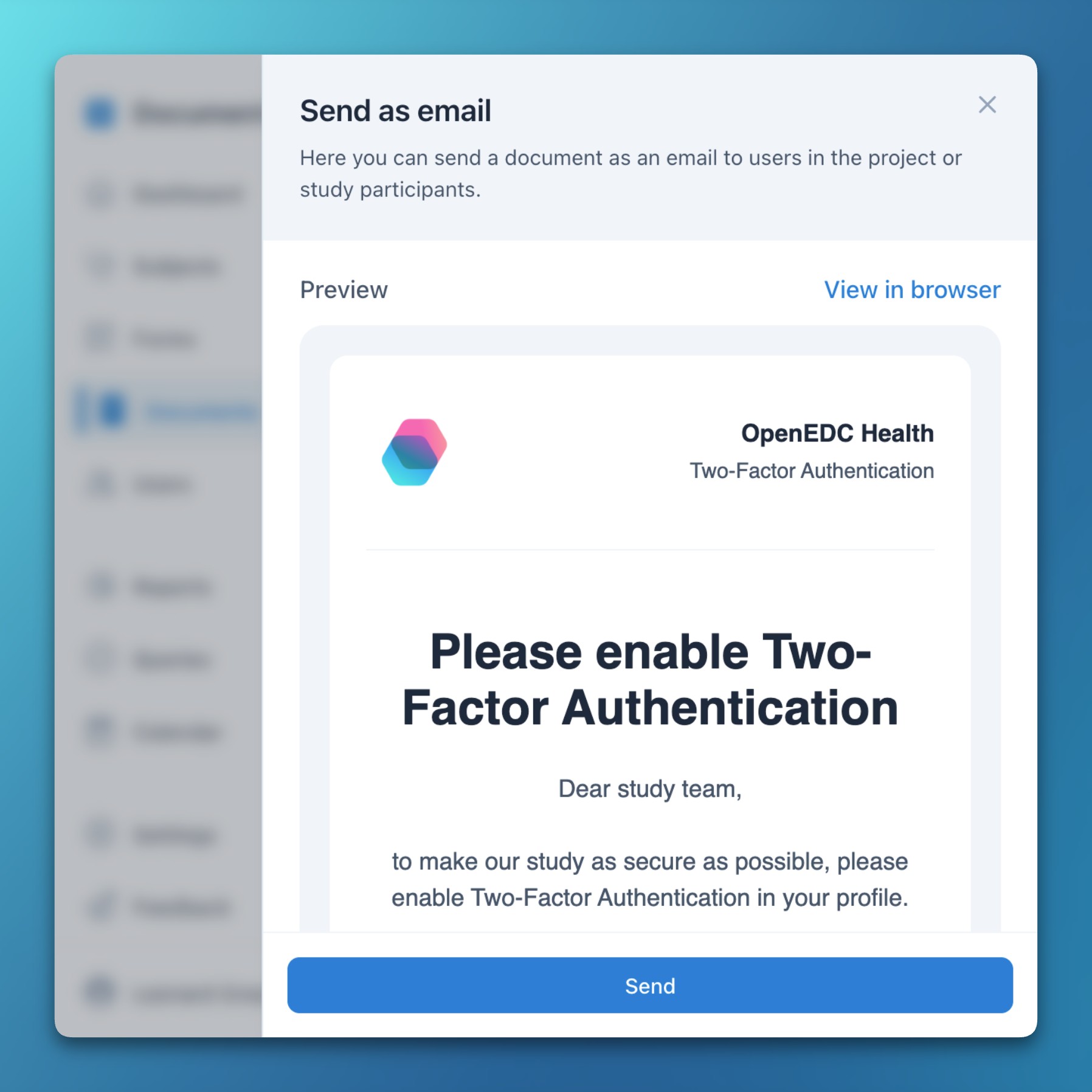
- Documents via email
- You can now send any document via email to your team members or study participants.
- This makes it easy to distribute important information like reminders or announcements in a timely manner.
- For each email, the subject, language, an optional redirect button, and the list of recipients can be individually configured.
Improvements
- Render translated expressions in descriptive texts in the currently selected language of a multilingual form instead of the app language.
- Improve the position of the range slider value when the field has a description and allow calculating it with an expression.
- Enhance spacing and layout in PDF/A exports for descriptive texts and documents with complex HTML content like nested lists.
- The CDISC ODM exporter and importer now support missing reasons and validation states for individual item data values.
Bug fixes
- Solved an issue where saving a form temporarily as incomplete would not work when a mandatory field in a data matrix was unanswered.
v2.20.0 (2025-09-05) Minor
Improvements
- The expression editor now automatically adds missing spaces before compound operators.
- Autocomplete items in the expression editor are now better sorted and grouped by event and form.
- The CSV clinical data import wizard now allows for re-mapping of text and numeric (i.e., non-codelist) values.
Bug fixes
- Solved a focus issue when clicking the expression editor below the actual input area not focusing the editor.
- Fixed a race condition where opening the dropdown menu next to a field (to add a new field below) and then opening the field’s options slideover (without adding a new field) caused the current field to lose focus, potentially resulting in unintentionally editing the parent element.
- Fixed another race condition when editing a form draft while having the same draft open for a subject (e.g., during initial database setup), which could result in the form getting stuck and not rendering properly.
v2.19.0 (2025-09-02) Minor
Improvements
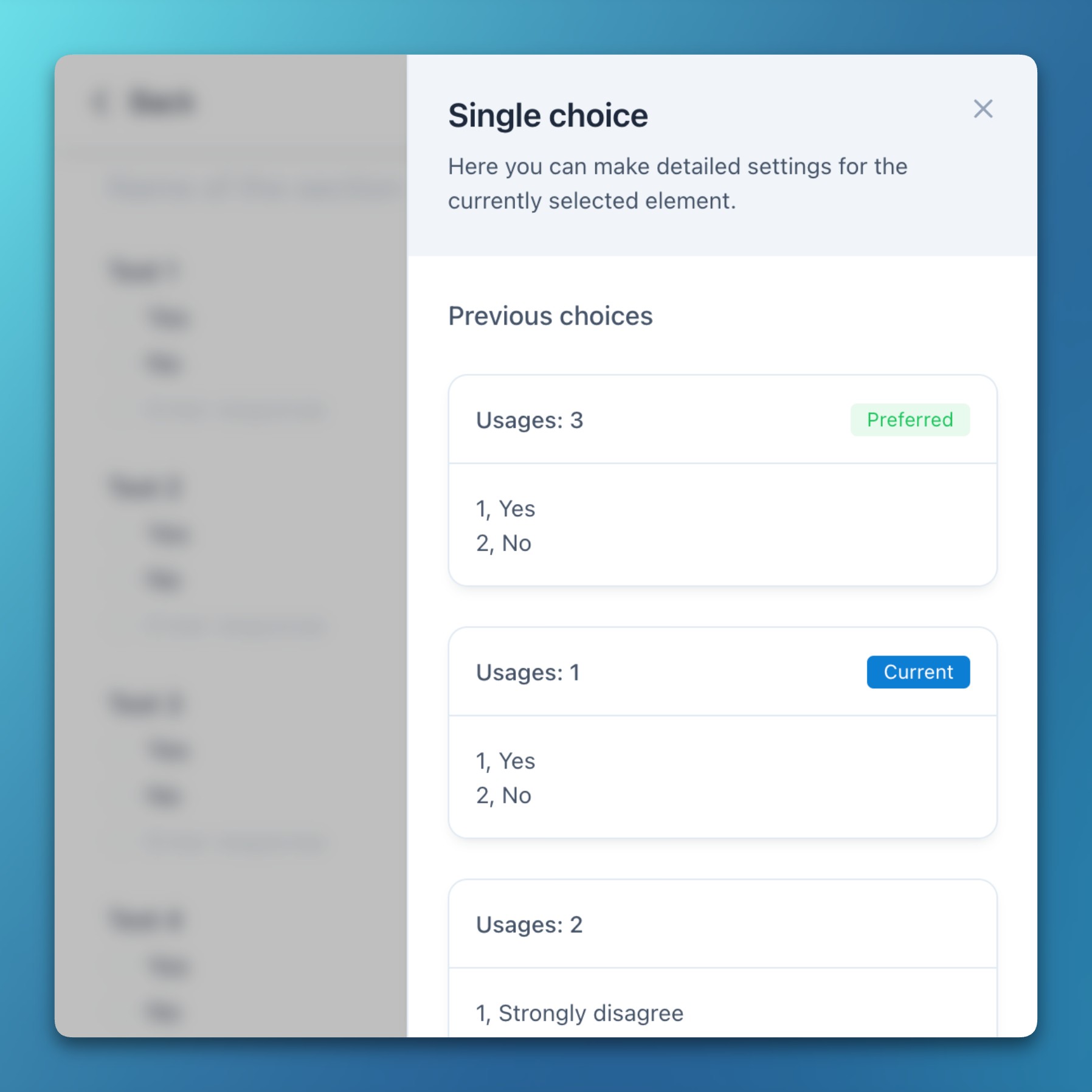
- Previous codelists are now grouped by coded values and sorted by the number of references, while also suggesting identical values with more references for improved standardization.
- When a study event or form is open, and a search input results in exactly one matched subject, the found subject will now be opened without closing the current study event or form.
Bug fixes
- Solved an issue that resulted in list filters not being project-scoped and thus still being active after switching to another project.
- Fixed a rare error with forms having more than 200 referenced items in expressions, resulting in an HTTP 414 (URI Too Long) response.
v2.18.0 (2025-08-21) Minor
Features
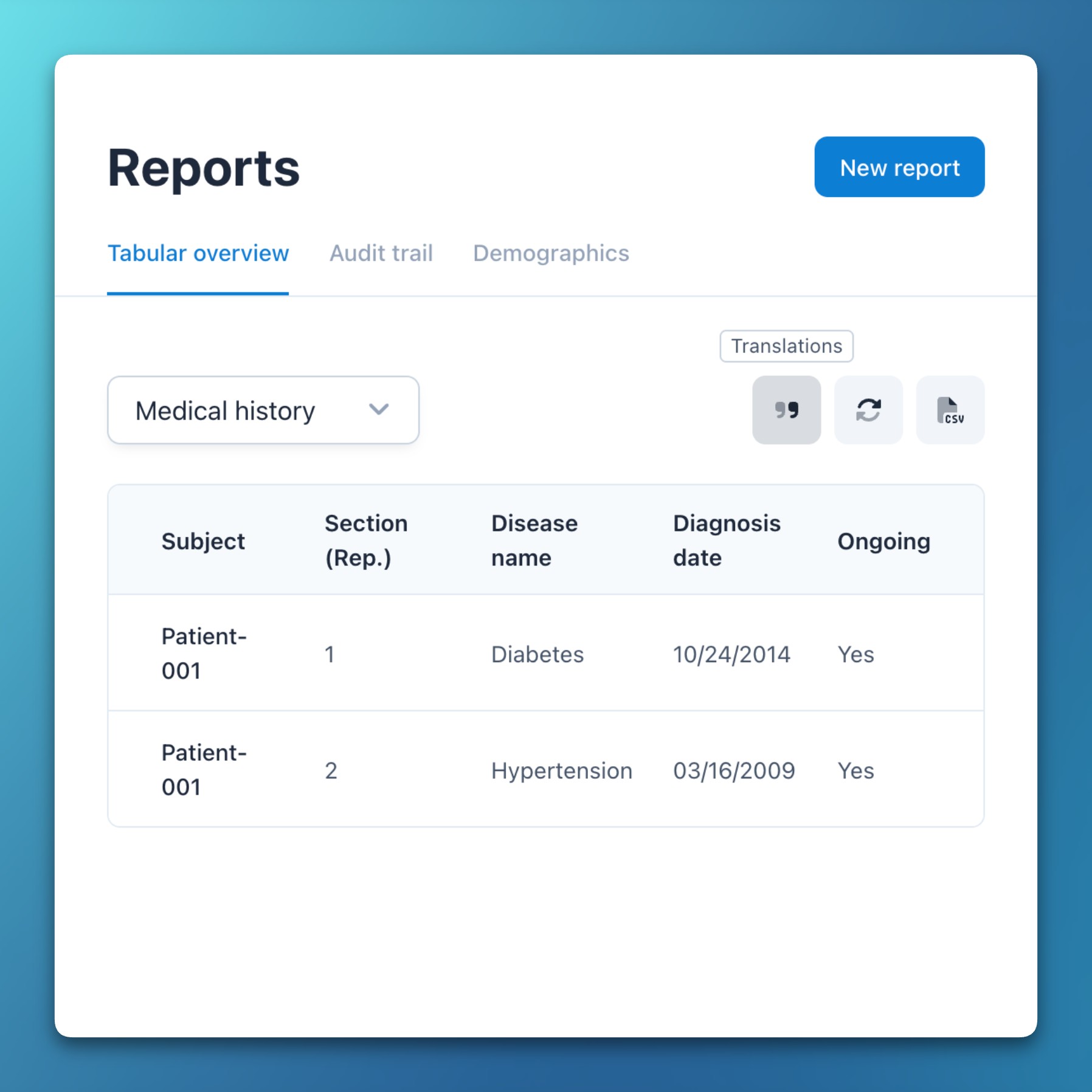
- Variable name rendering
- Added new project settings to explicitly choose whether variable names or translated texts should be rendered in conditions and reports.
- A new option in the default report allows to quickly switch between variable names and translated texts.
Improvements
- Removed option for mandatory fields from settings and instead make all new fields mandatory by default.
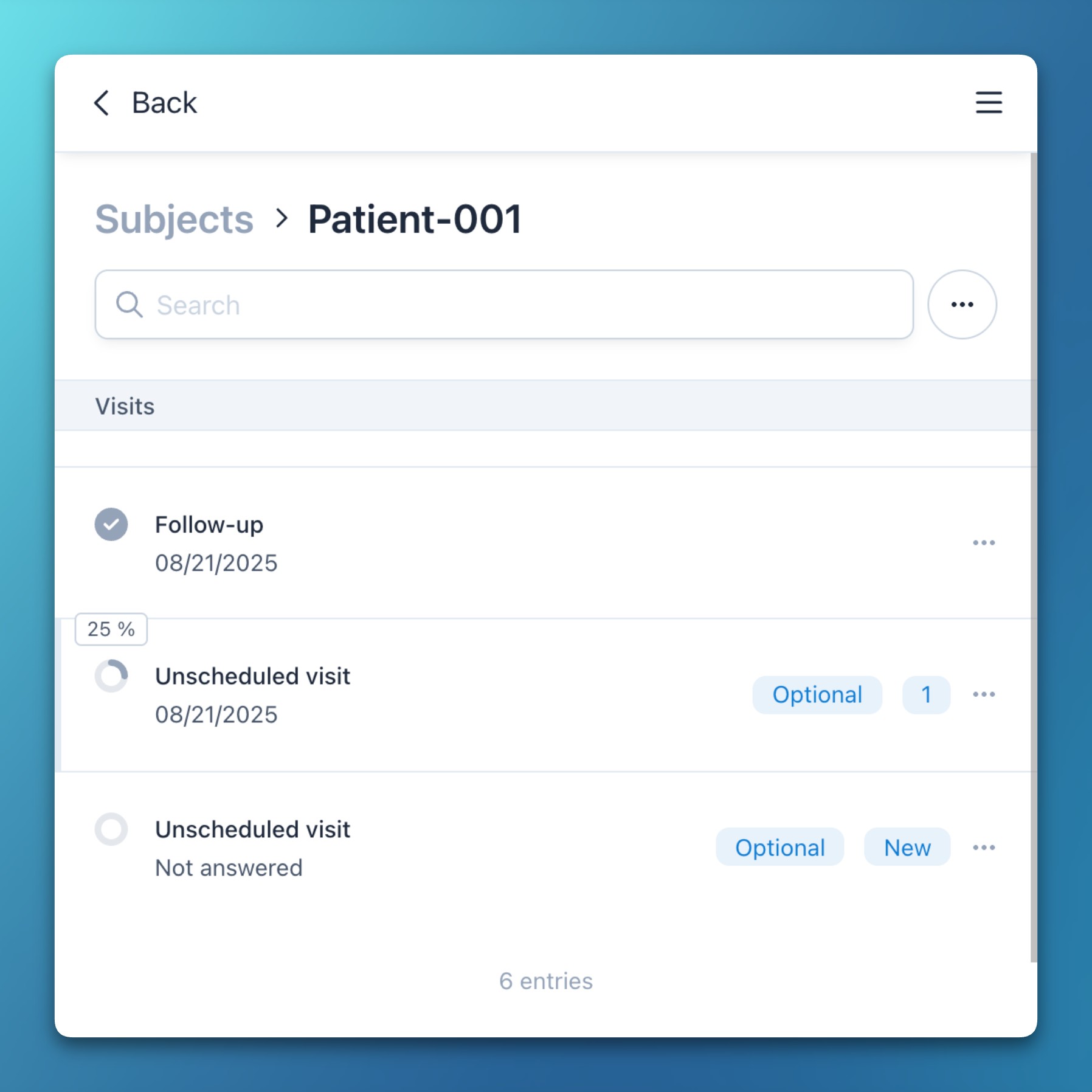
- Show the subject completion progress as percentage in a tooltip when hovering over the status icon.
Bug fixes
- Solved a rare issue when working in a server environment with a high latency (e.g., from another country) and rapidly creating new codelist items in the form editor, resulting in new items sometimes being overwritten.
v2.17.0 (2025-08-18) Minor
Features
- Expression enhancements
- Added a new environment variable to expressions, allowing fields to be hidden based on whether forms are answered in the app or survey.
- Added a new getParameter function to expressions, allowing access to URL parameters for dynamic form behavior or data retrieval.
- Besides the existing floor and ceil functions, added a new round function for rounding to the nearest whole number.
- Calculations can now be of type Preload (in accordance with CDISC ODM MethodDef) to calculate a field value only once and prevent it from being overwritten when form data changes.
Improvements
- Added text align justify option to documents and descriptive texts which is also supported in PDF/A exports.
Bug fixes
- Solved an error that prevented users from unselecting a date value by clicking on the same day in the calendar again.
- Fixed an issue where conditional fields in the application settings were sometimes not rendered correctly.
v2.16.1 (2025-08-14) Patch
Improvements
- Added border and center options to the Likert scale field for improved visual customization.
Bug fixes
- Fixed an issue where the button group field sometimes did not reflect data updates.
v2.16.0 (2025-08-13) Minor
Features
- Likert scales
- OpenEDC now provides a dedicated Likert scale field type, complete with a simple and interactive editor.
- Visually, a Likert scale is similar to a data matrix (introduced in v2.0.0), but offers a streamlined creation process.
- From a technical perspective, Likert scales are grouped items, enabling multiple Likert scales within one form section.
- This technical foundation opens up possibilities for future multidimensional data collection fields, such as coordinate systems.
- Likert scales are fully supported in PDF/A exports for robust and long-term and archival.
- Recode and average functions
- Added new recode function to remap coded values of multiple fields using a dictionary, enabling complex data transformations in calculations.
- Added new average function to calculate average values across multiple fields.
- These functions work seamlessly together to calculate scores for psychological questionnaires and scoring systems (e.g., SF-36).
Improvements
- To help distinguish between different types of validation, permissive (soft) validation messages are now displayed in blue, while strict (hard) rule violations remain in red.
- Improved performance when loading forms with many expressions that require data from other forms by fetching items in a batch.
- Active quick filters in lists are now highlighted in blue for better recognizability.
Bug fixes
- Fixed an issue where the form status could erroneously switch to Complete if a repeating section (with more than one instance) contained unanswered mandatory fields.
- Corrected a bug where removing a codelist item (i.e., a single or multiple choice option) sometimes didn’t work when using the backspace key.
- Solved a data export error in Chrome by requesting the directory handle before data fetch operations that could invalidate the user interaction context.
- Resolved a permission issue where project owners with minimal rights were not able to update project settings.
- Fixed two issues with conditions that sometimes prevented them from working in surveys or when referencing event names.
v2.15.2 (2025-07-28) Patch
Improvements
- Custom fields with codelist items (introduced in v2.12.0) are now rendered as quick filters in the subject list by default.
Bug fixes
- Fixed a bug since Chrome and Edge 137 (June 2025) where creating a new codelist item in the form editor sometimes caused the cursor to move up instead of down.
- Custom fields in documents or descriptive texts that evaluate to a numeric zero (0) are now rendered correctly.
v2.15.1 (2025-07-24) Patch
Improvements
- Improved the positioning and spacing of headings and lists in PDF/A exports.
- New study events are now always added to the last study event group for better organization.
- The select field dropdown menu now covers the entire width of the field.
Bug fixes
- Date and time fields that included descriptions were sometimes not properly aligned.
v2.15.0 (2025-07-23) Minor
Features
- Queries in CDISC ODM and PDF/A exports
- Queries can now be exported to, and imported from, CDISC ODM-XML files.
- Exports include the entire history of each query, with all comments and state changes.
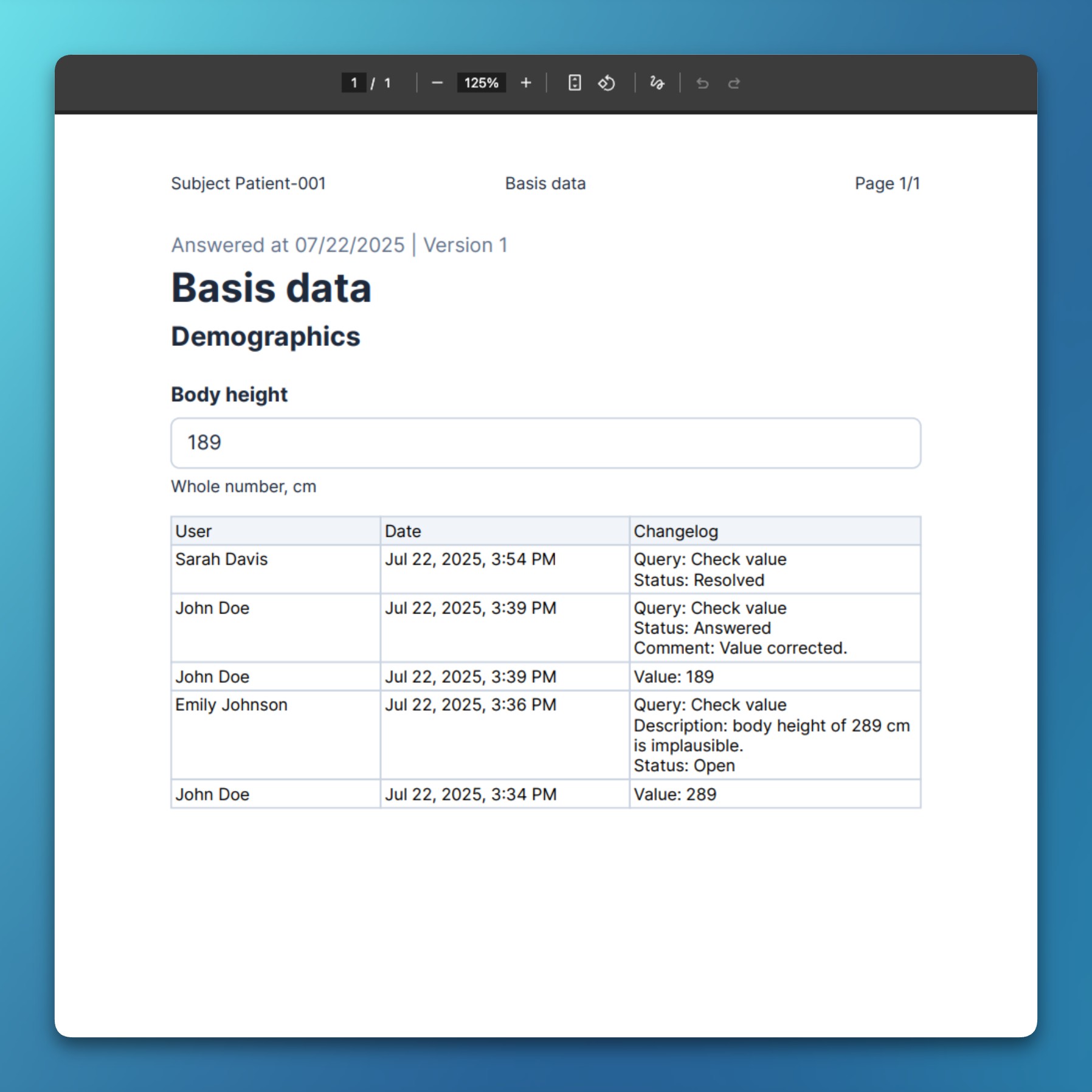
- For PDF/A exports, queries are added to an item’s audit table to provide a holistic overview.
Improvements
- When exporting a report table that has file columns, it’s now possible to export all included files as a directory.
v2.14.0 (2025-07-18) Minor
Features
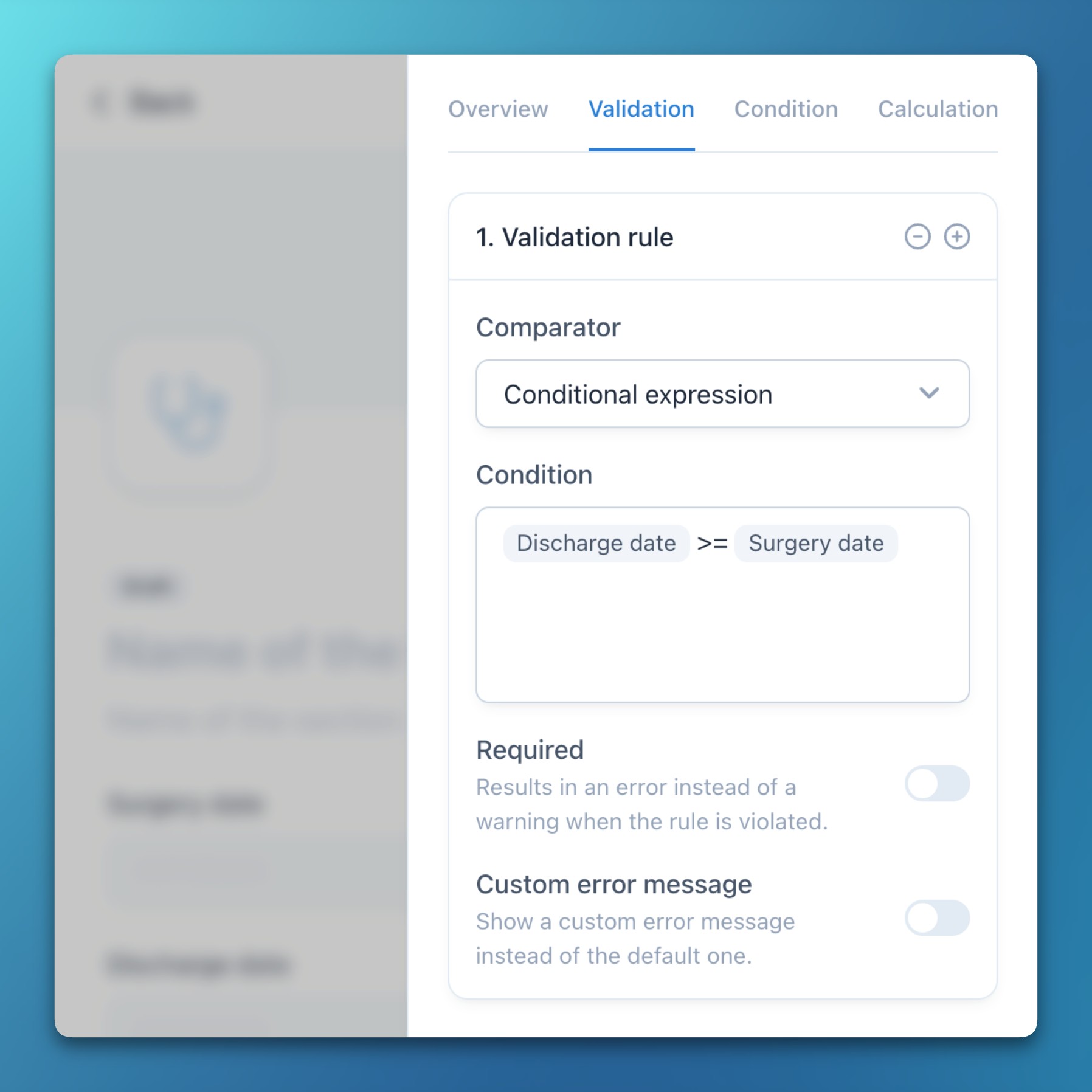
- Expressions in validation rules
- Custom conditional expressions can now be used in data validation rules.
- This allows for complex comparisons with previous values or functions (e.g., to ensure a date is not in the future).
- Furthermore, it is now possible to define custom and localized error messages for more precise user information.
Improvements
- Start and end of line anchors in regular expression validations are now appended implicitly, simplifying the process.
v2.13.1 (2025-07-15) Patch
Improvements
- Repeating event, form, and section instance numbers are now rendered more consistently.
- The study protocol and event groups have been added to the audited data models.
Bug fixes
- Document updates are now rendered correctly when only the name of the document is changed.
- Fixed an issue where query updates that only contained a comment but no data change were not rendered.
v2.13.0 (2025-06-26) Minor
Features
- Redesigned documents
- The document editor now supports text alignment and tables.
- A new fixed inline menu is always reachable at the top of a draft document.
- Documents can now be exported to PDF/A, including text alignment, tables, images, lists, and more.
- Expressions in documents
- It’s now possible to add arbitrary expressions to documents and descriptive texts.
- Expressions can pipe in previously entered (clinical) data or perform calculations.
- New functions in expressions help to format dates and numbers into localized values.
- Conditional sections in documents can be hidden based on previous inputs.
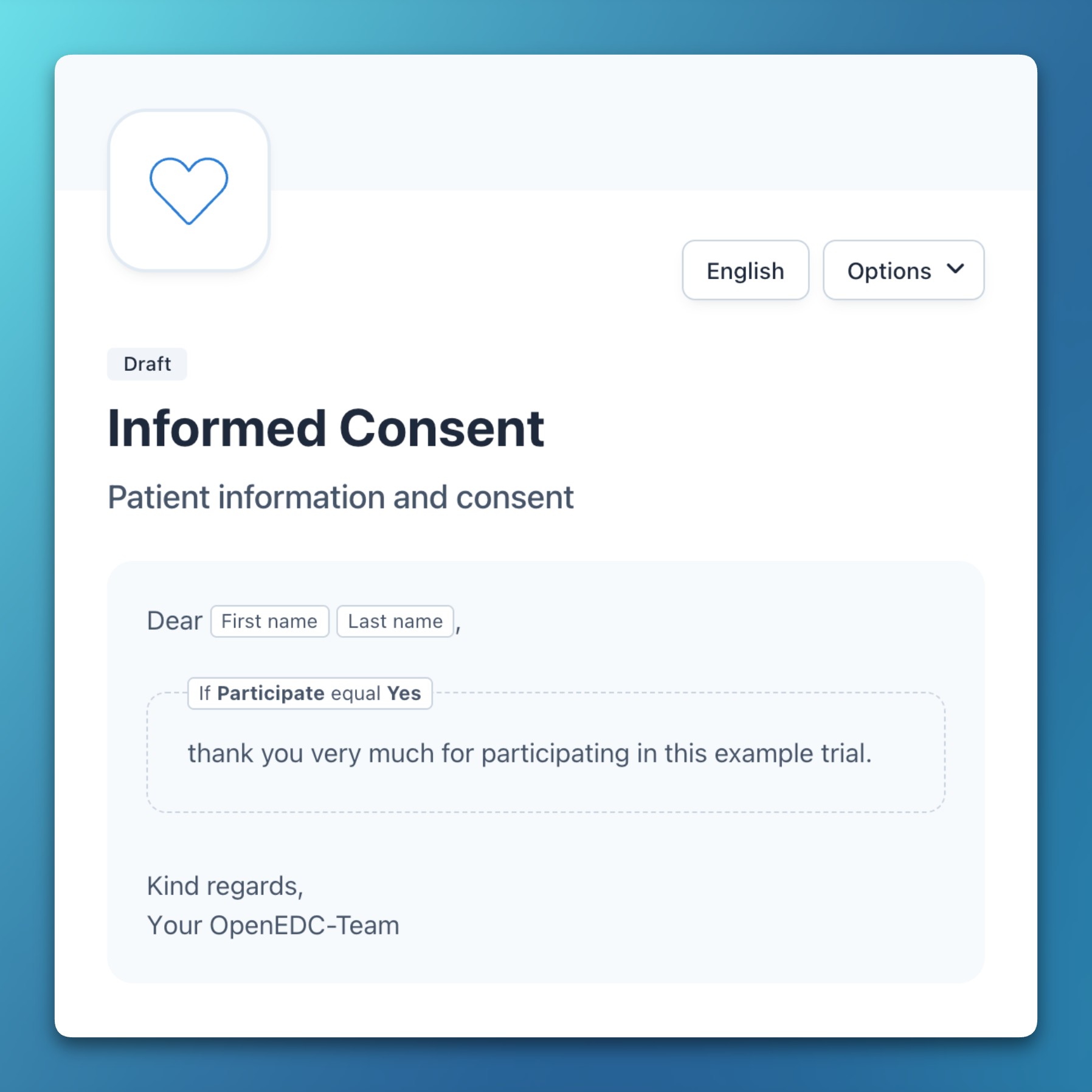
Improvements
- Custom fields are now rendered for events and forms when available.
- In addition to fields, sections, forms, and visits, study event groups can now be re-ordered via drag-and-drop.
v2.12.0 (2025-06-07) Minor
Features
- Custom fields in subject list and table
- You can now display any form field as a custom value in the subject list and table.
- Subjects can be filtered and sorted by these custom fields.
- The default subject creation date can be hidden if desired.
- This enables use cases such as adding a custom subject status or displaying an alternative date field.
Improvements
- For fields in expressions (conditions and calculations), the event name is now shown if it’s different from the current one.
- Added custom step intervals for numeric inputs (integer and decimal), as well as for the range slider.
- The aggregated subject form status now renders more granular values instead of 20% steps.
- Numeric fields now use region-specific decimal separators in reports and exports.
Bug fixes
- Temporarily disabled the automated inactivity logout during large data imports to prevent interruptions.
- Improved the handling of conditional forms in repeating events.
- The completion progress for optional events is now shown without influencing the aggregated subject status.
v2.11.0 (2025-05-26) Minor
Features
- Delete form data
- For each subject, a new menu option allows you to delete all data and the status of a selected form.
- For repeating forms, the selected instance will also be removed.
- Deletion requires permission and is recorded in the audit trail.
- Required two-factor authentication
- The owner of a project can now mandate two-factor authentication (2FA) for all users.
- If a user without 2FA enabled attempts to access such a project, they will see an error with an option to enable it.
Improvements
- When a form is unselected from all events in the assignment matrix, an error message now prevents losing the reference to it.
- Improved the performance of file uploads, downloads, and previews.
v2.10.0 (2025-05-19) Minor
Features
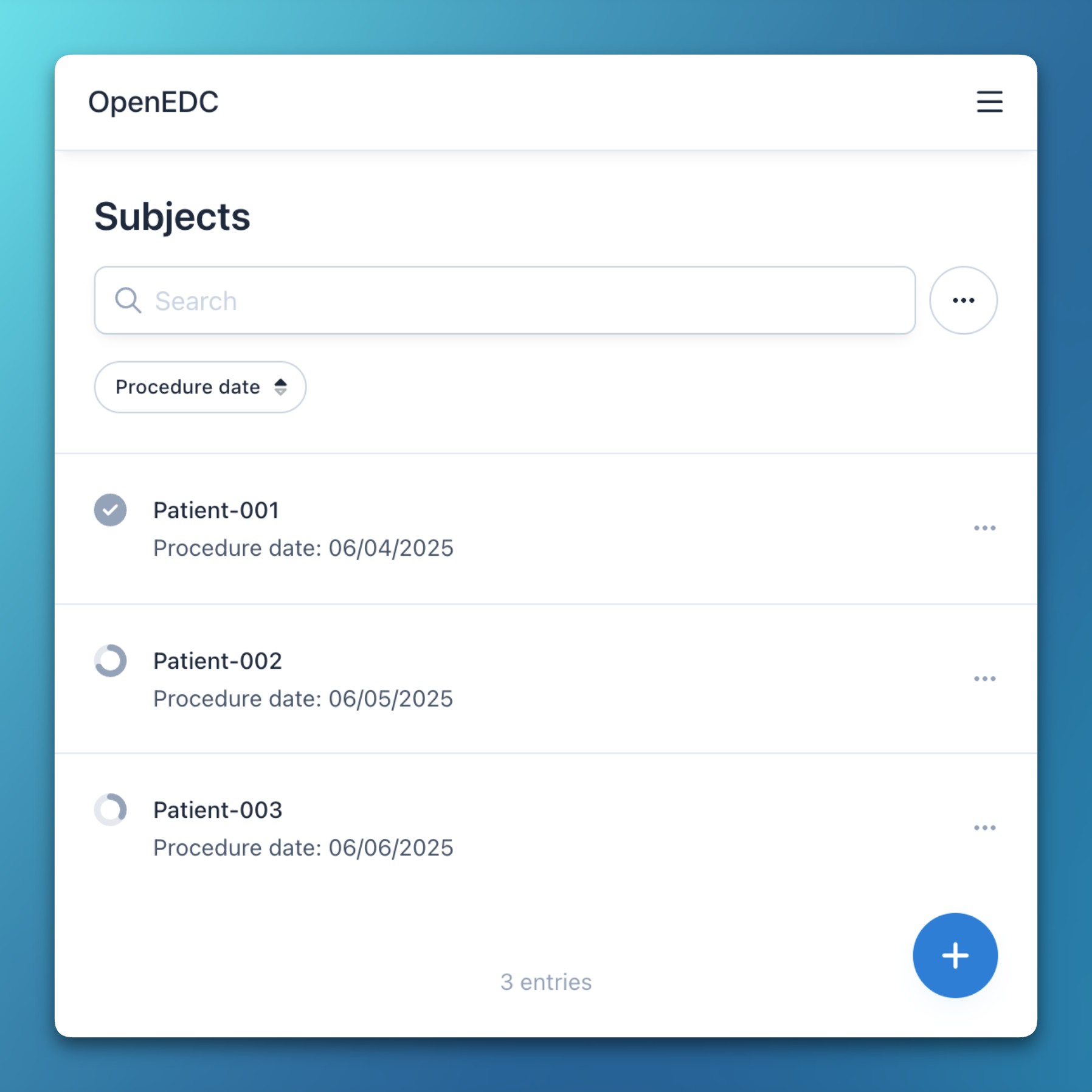
- Sortable subject list
- Subjects in the list view can now be sorted by their creation date in either ascending or descending order.
- Additionally, the list’s sort, filter, and search states are now persisted between navigations and reloads.
Improvements
- Subjects can now be filtered by their status: incomplete, complete, verified, or locked.
v2.9.0 (2025-05-16) Minor
Features
- PDF export and print functionalities now support including the audit trail.
Improvements
- Improved and extended the help documentation, especially for data management topics.
- In addition to events, forms can now also be marked as optional, which influences status aggregation.
- Added list and table virtualization for improved performance when rendering thousands of subjects.
v2.8.1 (2025-04-11) Patch
Improvements
- The guided CSV import now supports multiple choice data that is comma-separated.
- The CSV import now also supports repeating events, forms, and sections.
v2.8.0 (2025-04-07) Minor
Improvements
- File uploads are now resumable, show upload progress, and support files of up to 5 GB.
- The form header now supports custom image uploads and is resizable.
Bug fixes
- During a CSV import, the mapping of default fields sometimes did not work reliably.
v2.7.4 (2025-03-23) Patch
Improvements
- The range slider field now supports the decimal data type, which is useful for things like percentage values.
Bug fixes
- For timezones behind UTC, plain dates were sometimes rendered with a negative offset of one day.
- When auto-incremented keys were enabled, the subject slideover wasn’t shown, even when a user was assigned to multiple locations.
v2.7.3 (2025-01-24) Patch
Improvements
- Enhanced the performance and user interface of the form versioning dialog.
- Icons are now hosted with the app to improve performance through regional hosting.
Bug fixes
- You will now be correctly redirected to a subject from a scheduled event calendar entry.
v2.7.2 (2024-12-29) Patch
Bug fixes
- The item question of relative paths in conditions will now always be rendered.
- Ensured that zeros are considered as a value in comparative expressions.
- When opening a form from a query, the affected field will now be scrolled into view.
v2.7.1 (2024-12-21) Patch
Improvements
- Made performance improvements for queries.
Bug fixes
- Forms are now hidden in the status overview for users without edit or view permissions.
- The “add event” button is now hidden for users without editing permissions.
v2.7.0 (2024-12-20) Minor
Features
- Form status overview
- The subjects module now offers a tabular overview of all form status values: incomplete, complete, verified, locked, and skipped.
- When clicking on any status, a slideover appears for data entry or editing.
- When visits or events are enabled, grouping headers visually join all forms from one event.
- Fixed headers and the subject key column allow for precise identification even when scrolling in large tables.
- Header sorting options allow you to sort by subject key, recruitment date, and location.
- New repeating instances for events and forms are rendered dynamically.
v2.6.1 (2024-12-20) Patch
Bug fixes
- When clicking on the top right arrow within a query, not only the event but also the corresponding form will now be opened.
- On a CDISC ODM import, data UUIDs will now be replaced to ensure uniqueness.
- When creating a new form draft, multiple references to the same codelist are no longer duplicated.
- In conditions with boolean operators, numeric values are no longer compared using lexicographical order.
v2.6.0 (2024-12-06) Minor
Features
- Pie chart
- A new pie chart can now be added to custom reports to visualize the ratio of categorical values.
- A chart-based filter can now be deselected by simply clicking on the value again.
- Role and permission matrix
- Roles and their assigned permissions can be viewed and edited in a tabular overview for improved clarity.
- Two new default roles were added for study nurses and data managers.
- Date binning in charts
- It’s now possible to group date values by year, month, day, or weekday in categorical charts (e.g., bar or pie charts).
- Binned values can be used as live filters (e.g., showing subjects recruited in a specific year, month, or a combination of both).
Improvements
- The date picker now also supports the time-only data type.
v2.5.3 (2024-11-18) Patch
Improvements
- Authorized users may now withdraw their own signatures.
- Based on locations and permissions, only relevant users are displayed in the list of responsible people for a query.
v2.5.2 (2024-11-17) Patch
Improvements
- The form lock state can now be set independently from the completion and verification status.
- This change also fixes an issue where the form status button was disabled for users who had lock-forms permission but not edit-data permission.
- Form signatures are now rendered in the audit trail slideover within the subjects module.
v2.5.1 (2024-11-12) Patch
Improvements
- The form status aggregation now only considers mandatory events and shows the completion progress as a percentage.
- Improved authentication routing by using One-Time Passwords (OTPs) instead of URLs for invitation and recovery emails.
v2.5.0 (2024-11-03) Minor
Features
- Bulk signatures
- Users may now sign (and optionally lock) all forms of a given subject or event at once.
Improvements
- For long forms, the footer now floats at the bottom of the screen for quick access to save and close options.
- An error is now shown when a user attempts to assign an already occupied variable name to an element.
- User roles can now be exported and imported in the CDISC ODM format.
- An item may now have multiple validation rules (i.e., range checks) assigned to it.
v2.4.0 (2024-10-23) Minor
Features
- Open and logout (tablet mode) for surveys
- Surveys can now be opened directly from within the app, which will first log out the current user.
- After the survey is finished, a link is rendered to redirect the user back to the app, which will require a new login.
- The redirect search parameter can also be used for custom purposes in self-embedded surveys.
- Study arms
- Study event groups can now be defined as arms, with customizable and translated descriptions.
- Subjects must be explicitly assigned to a study arm in order to collect data for that arm.
- Conditional forms
- In addition to events, sections, and items, conditional expressions can now also be attached to forms.
- Events and forms with unmet conditions are still rendered in the subjects list but are disabled for editing.
- A notification is shown for each skipped form, explaining why it has been conditionally disabled.
Improvements
- Implemented batched clinical data persistence for improved performance.
v2.3.0 (2024-10-14) Minor
Improvements
- Range checks can now be defined as “soft” or “hard,” where soft checks only give a warning instead of preventing data from being saved.
- A regular expression can now be specified in the settings to define and constrain the format of manually entered subject keys.
- Added a new permission that is required for a user to be able to delete subjects.
- Support for custom translations has been added via an addTranslationOverwrite() API.
- There is a new option to batch export multiple CSV files, split by event and form.
- A new permission allows forms to be locked independently of their verification status.
- Events can now be used within conditional expressions to hide or show fields based on the current event.
- You can now revoke the verification of item data via the status panel.
Bug fixes
- Inputs in the data matrix are now correctly disabled if a user is missing edit permissions.
- The form status selection is now properly enabled for users with verify and sign permissions.
v2.2.0 (2024-09-23) Minor
Features
- Reuse of codelists
- The codelist panel now renders previously defined codelists and allows you to reuse them.
- Codelists are reused by reference rather than by copying them, and the number of references is shown for each codelist.
- File uploads in documents
- The documents module now allows the upload of files while rendering PDFs, images, and videos.
- Versioning is supported by uploading new file versions for a specific document.
- When referenced in forms, supported files are rendered there as well.
Improvements
- Reports and CSV exports now render event and form names in dedicated columns and only include each item once, even if it is referenced in multiple events or forms.
v2.1.0 (2024-09-04) Minor
Features
- Pattern matching
- For fields with a text data type, a regular expression can be specified to constrain the data input.
Improvements
- Mandatory fields are now clearly labeled in the PDF export.
- Added support for the datetime data type in a data matrix.
- Data matrices are now validated on input and on submit.
- Added abs, date, left, and right functions to the expression parser.
Bug fixes
- Added a missing project field to the compound key path.
- The status for forms with multiple sections is now aggregated properly.
- For autocompletions in expressions, the field list will now be correctly returned for items that do not have a codelist.
v2.0.0 (2024-08-27) Major
Features
- Data matrix
- Form sections can be enabled as a “data matrix” to render inputs in a tabular view.
- Repeating sections can dynamically add and delete rows for repeating instances.
- Subsequent sections with the same or similar questions are merged into one table.
- When required, a matrix can be temporarily transposed to edit queries and perform SDV.
- Trial protocol PDF export
- There is a new option to print all events, including all forms of a project.
- This feature supports repeating events, forms, and sections, even if they are nested.
- It also supports printing all clinical data for a selected subject.
- You can now optionally add variable names and coded values to the PDF export.
Improvements
- Compound primary keys
- Item data and form status models now overwrite existing data on API imports.
- Data imports are now performed in batches to improve performance.
- Batched transactions
- Persisting hierarchical data now uses batched database transactions to improve performance.
- Unified CSV and PDF exports
- In addition to the report module, CSV files can now be downloaded from the export module.
- In addition to the forms and subject modules, PDF files can now also be downloaded from the export module. This uses the new File System Access API when PDFs for multiple subjects need to be downloaded.
v1.9.5 (2024-08-10) Patch
Improvements
- Single choice fields can now be visually represented as select or dropdown menus.
- Forms can now be saved with missing mandatory fields when the status is set to “incomplete”.
- The use of special characters and spaces within subject keys is no longer prevented.
- Added support for count() and sum() in calculation and conditional expressions.
Bug fixes
- Fixed accessibility issues with the range slider field on mobile Safari (iPadOS and iOS).
v1.9.4 (2024-08-09) Patch
Improvements
- In addition to events and fields, conditional expressions can now be entered for sections.
- Study events can now be assigned to pre-defined groups (currently general and visits).
v1.9.3 (2024-08-05) Patch
Improvements
- Adding existing forms to an event can now be done via a tabular view, which shows the assignment for all events.
v1.9.2 (2024-08-03) Patch
Improvements
- In addition to forms and sections, events can now be set as repeating.
v1.9.1 (2024-08-02) Patch
Improvements
- Added support for date subtraction, as well as floor() and ceil() functions, in expressions for calculated fields.
- A user’s preferred name and email are now copied when adding a non-centrally managed user to another project.
Bug fixes
- Numeric expression results for calculated fields of the “integer” type are now rounded correctly.
- The translated missing reason is now rendered correctly in forms and reports.
v1.9.0 (2024-07-25) Minor
Features
- Calculated fields
- Mathematical expressions can now be used to automatically calculate fields (e.g., to calculate the BMI).
- The expression editor was updated to suggest mathematical operators (such as for add, subtract, multiply, and divide).
Improvements
- Decimal fields are now rendered with two decimal places by default.
v1.8.0 (2024-07-23) Minor
Features
- End-to-end encryption for clinical data
- Text, date, and signature fields now support end-to-end encryption for protecting personally identifiable information.
- The encryption uses the PRF extension for Passkeys, enabling biometric encryption via Touch ID, Face ID, or Windows Hello.
- A hybrid encryption algorithm uses asymmetric and symmetric primitives from the Web Crypto API (RSA-OAEP and AES-GCM), supporting end-to-end encrypted survey and participant data via a public project key.
- Encrypted data can be viewed after authentication within forms, reports, and the audit trail.
Improvements
- File uploads can now be viewed and downloaded from the audit trail.
v1.7.0 (2024-07-11) Minor
Features
- Metadata versioning
- Each form / eCRF now either has a version number or is in draft mode. Published forms can no longer be edited without creating a new draft first.
- When publishing a draft, the system checks already-captured clinical data for compatibility with the new draft and shows errors for any inconsistencies.
- PDF/A export
- Answered and empty forms, as well as entire events, can now be exported in PDF/A format.
- The export supports repeating forms and sections, conditions, electronic signatures, descriptive texts including images, and file upload fields.
- Export and import module
- For data exports, various options to select data are available, such as specific events, forms, locations, documents, and files.
- When importing CDISC ODM, these options are also available, allowing you to specifically select which data should be imported.
- Importing CDISC ODM now allows you to merge metadata into an existing project while showing already-existing events and forms.
Improvements
- The eConsent feature now uses metadata versioning to support arbitrary patient information documents, while showing the signed version for each subject.
- Translated conditions are now rendered directly in the editor fields.
- Customizable preset roles with a defined set of initial rights are now available in the user management module.
- The date picker field now supports the datetime data type.
v1.6.0 (2024-06-13) Minor
Features
- Participant app and self-registration
- Study participants can now register themselves via a survey and then use a mobile web application to answer scheduled events.
- The mobile application shows current, upcoming, and previous events and allows for editing previously made answers.
- Participants can use multi-factor authentication and other account-related functionality.
- The user module was updated to provide quick filters for showing team members or study participants.
- The user module now shows a default role for participants to select forms that may be viewed or edited.
Improvements
- A missing reason with an optional comment can now be specified for every field in a form at once.
- The query details now automatically scroll to recently made changes to improve collaborative editing.
v1.5.1 (2024-06-04) Patch
Improvements
- Automatically rendered upcoming event periods can now be specifically dated, timed, and persisted per subject.
Bug fixes
- All-day events will now always be rendered before timed events.
v1.5.0 (2024-05-28) Minor
Features
- A new calendar module is available for both collaborative and personal date management, supporting timed and multi-day events.
- You can now schedule events (or visits) with absolute or relative time periods, including the automatic rendering of upcoming events per subject in the calendar and subject modules.
v1.4.1 (2024-04-30) Patch
Improvements
- Network connectivity issues are now caught when submitting clinical data in the subject module and in surveys.
v1.4.0 (2024-04-29) Minor
Improvements
- An automatic inactivity logout feature has been added, which logs out a user after a defined period of inactivity.
- A user’s account will now be automatically locked after a defined number of successive failed authentication attempts.
v1.3.3 (2024-04-27) Patch
Improvements
- The web app now includes an interactive onboarding tour that introduces key features.
Bug fixes
- Expression data for nested conditional fields will now be unset rather than deleted.
v1.3.2 (2024-03-25) Patch
Improvements
- A cryptographic hash has been added to form signatures to securely identify a specific set of values.
- Multi-factor authentication is now allowed when signing forms.
v1.3.1 (2024-03-20) Patch
Improvements
- Significantly improved the performance for loading metadata from the server.
v1.3.0 (2024-03-13) Minor
Features
- There is now a default report to view the audit trail for all audited data models.
- The audit trail has been enabled for metadata and admin data models.
v1.2.0 (2024-03-08) Minor
Features
- Added form signatures with re-authentication to allow users to take legal responsibility for data.
v1.1.0 (2024-03-02) Minor
Features
- Implemented Source Data Verification (SDV) and Missing Reasons (Null Flavors).
- Uploaded files can now be previewed in reports.
- Implicitly given permissions are now shown visually.
- Data read and write permissions can now be specified on a per-form basis.