The - Modern
- Simple
- Secure
- Modern
Solution for Clinical Trials
A validated system for clinical trials with a focus on user-friendliness, collaboration and data standards. Fully GCP and GDPR compliant.
Locally on the Computer
Create professional, multilingual eCRFs — including real-time validations, branching logics, and repeatable elements. Invite team members with a role-based permission system. Conduct multi-center studies and track all changes in an audit trail.
Mobile on Tablets
Collect data on your computer or mobile devices, let participants sign versioned consent forms digitally, and access your studies flexibly from anywhere.
As an App for Patients
Send ePROs or use our dedicated patient app with push notifications for automatic reminders and improved engagement.


Complete Diary
Now
Dear patient, your next diary form is ready. Please tap here.

Our Cloud: Your Secure Platform for Clinical Trials.
OpenEDC is a modern, simple, and secure platform for clinical trials. Request a free demo.
Industries and Application Areas
From Patient Diaries to Clinical Trials
OpenEDC is the ideal solution for every industry and research project. From patient diaries, to in-hospital outcomes research, to multi-center clinical trials. The modular approach allows the solution to be customized individually.

Pharma and Biotechnology
OpenEDC supports pharmaceutical and biotech companies in conducting regulated clinical trials with a fully validated system. Our platform meets all requirements according to ICH-GCP, FDA 21 CFR Part 11, and EU-GMP Annex 11 for maximum data integrity and compliance.
Medical Device Manufacturers
Medical device manufacturers can efficiently conduct clinical investigations and post-market surveillance with OpenEDC. The system meets all regulatory requirements of the MDR and provides audit-proof documentation, electronic signatures, and a complete audit trail.
Clinical Research Organizations (CROs)
CROs benefit from a flexible, GCP-compliant EDC system for multi-center studies. OpenEDC offers a comprehensive role and rights systems, Source Data Verification, Query Management, and full validation according to GAMP 5 – for the highest quality in every project.
Hospitals and Institutes
University hospitals and research institutions can implement patient diaries, registries, epidemiological studies, and outcomes research with OpenEDC. The system enables multi-center projects, global user management, and meets all data protection requirements.
Features
A Variety of Opportunities
OpenEDC offers you all the essential functions to implement your research project quickly and easily.
A Modular Platform
From simple electronic data capture, through eConsent, to fully decentralized clinical trials: OpenEDC provides everything that modern medical research needs today. Features can be combined freely to conduct any study efficiently and securely. Click on a feature to learn more.
 OpenEDC
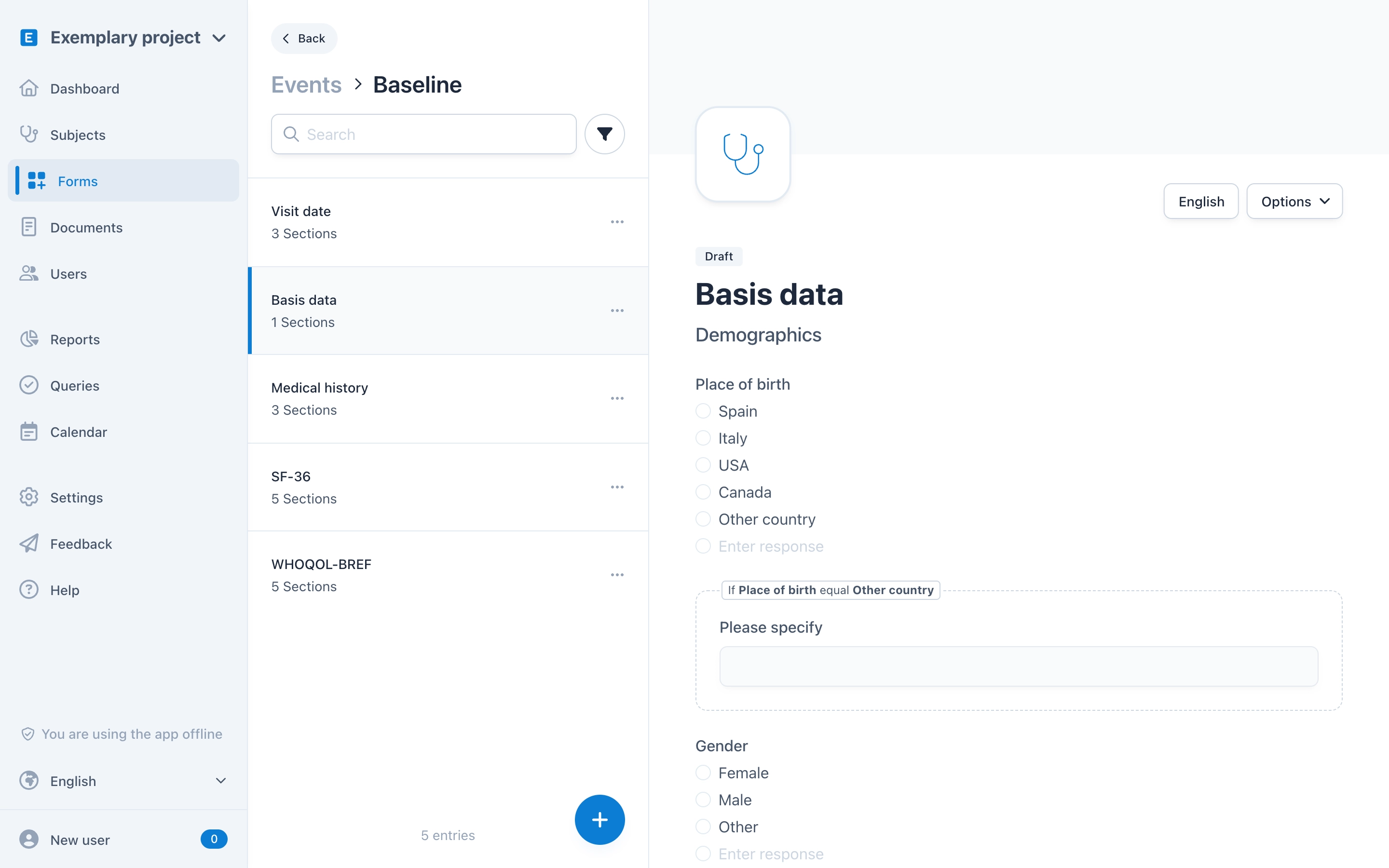
OpenEDC Simple Form Creation
OpenEDC offers an interactive, easy-to-understand form editor. Type in questions and pre-defined answer options in a natural way. See what the finished form looks like at any time. Create multilingual forms with real-time validations, branching and conditional logics, file uploads and much more.
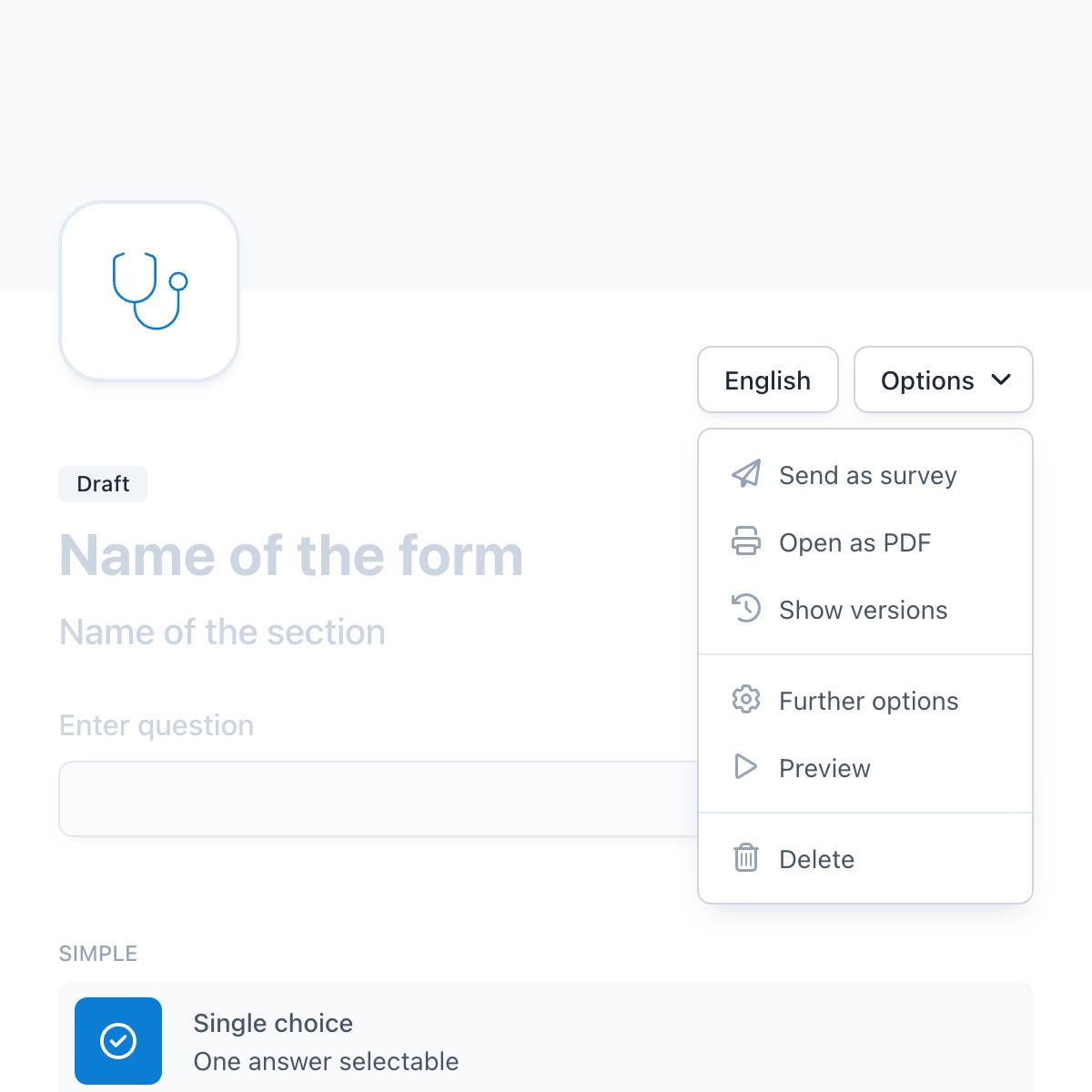
Reports and Insights
OpenEDC allows you to create powerful, highly interactive reports. Simply filter data with one click and combine multiple filters until you see the data selection you want. You can then easily export this selection, analyze it further with other statistical tools or share it with colleagues.
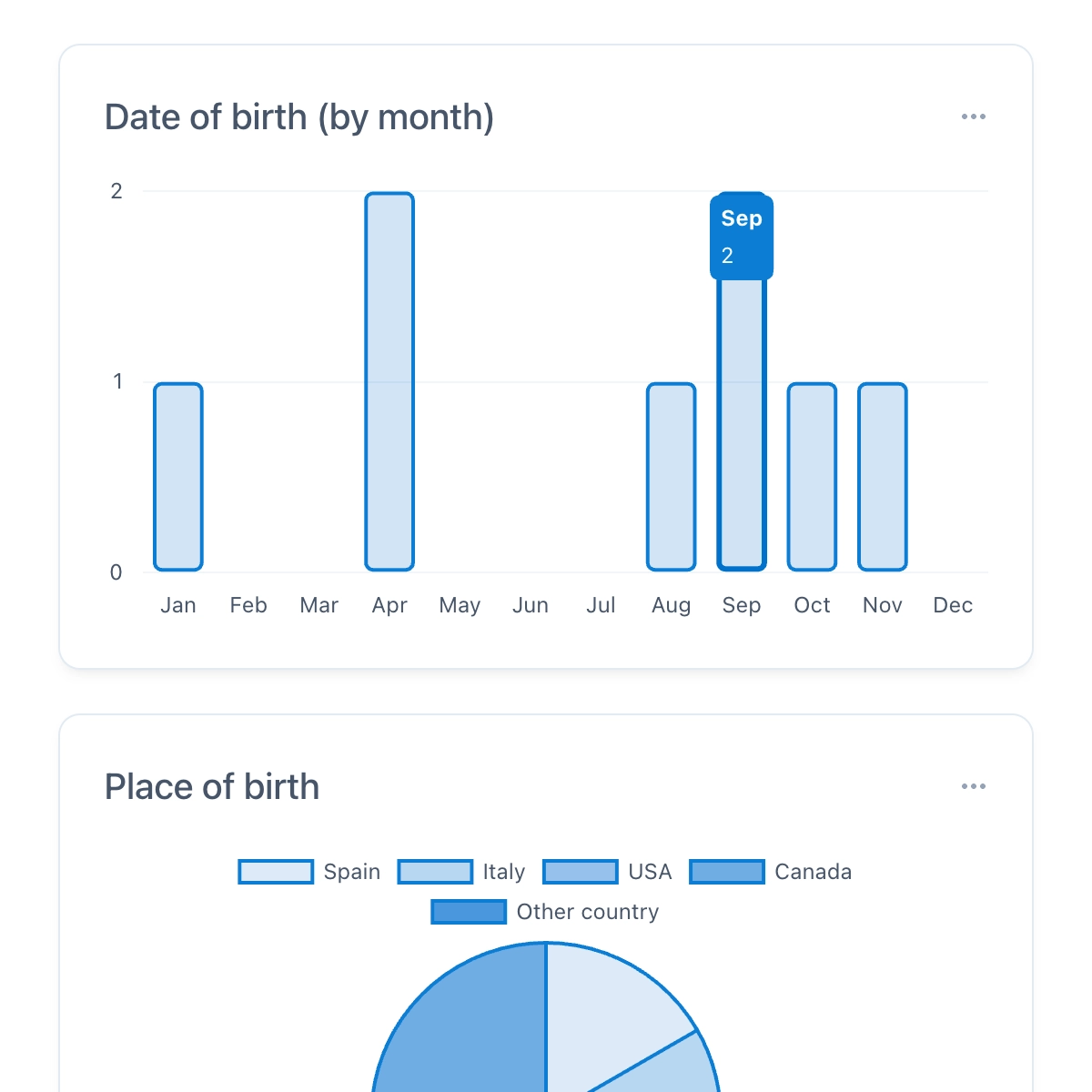
FDA, GCP, and EU Compliant
OpenEDC complies with all legal requirements according to FDA 21 CFR Part 11, ICH-GCP, and EU-GMP Annex 11. For example, the audit trail automatically logs all changes with a timestamp, electronic signatures require re-entering the password, and the role and rights system allows selective access restrictions.
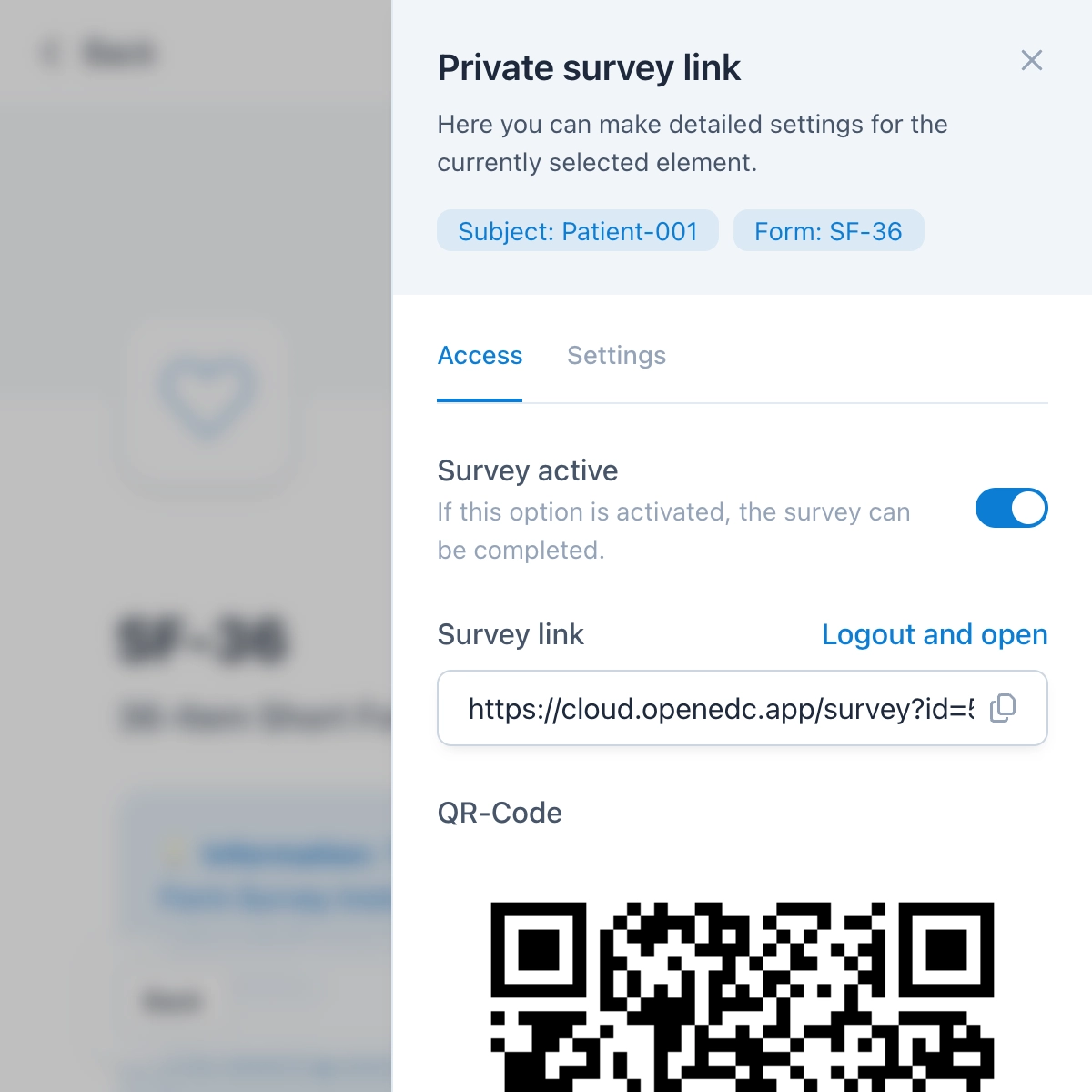
Surveys for ePRO and eCOA
You can send surveys as a link or QR code in a matter of seconds and thus collect electronic patient-reported outcomes (ePROs or eCOA). Additionally, you can give participants the opportunity to register themselves for the study, after which they can use a dedicated participant app for upcoming visits.
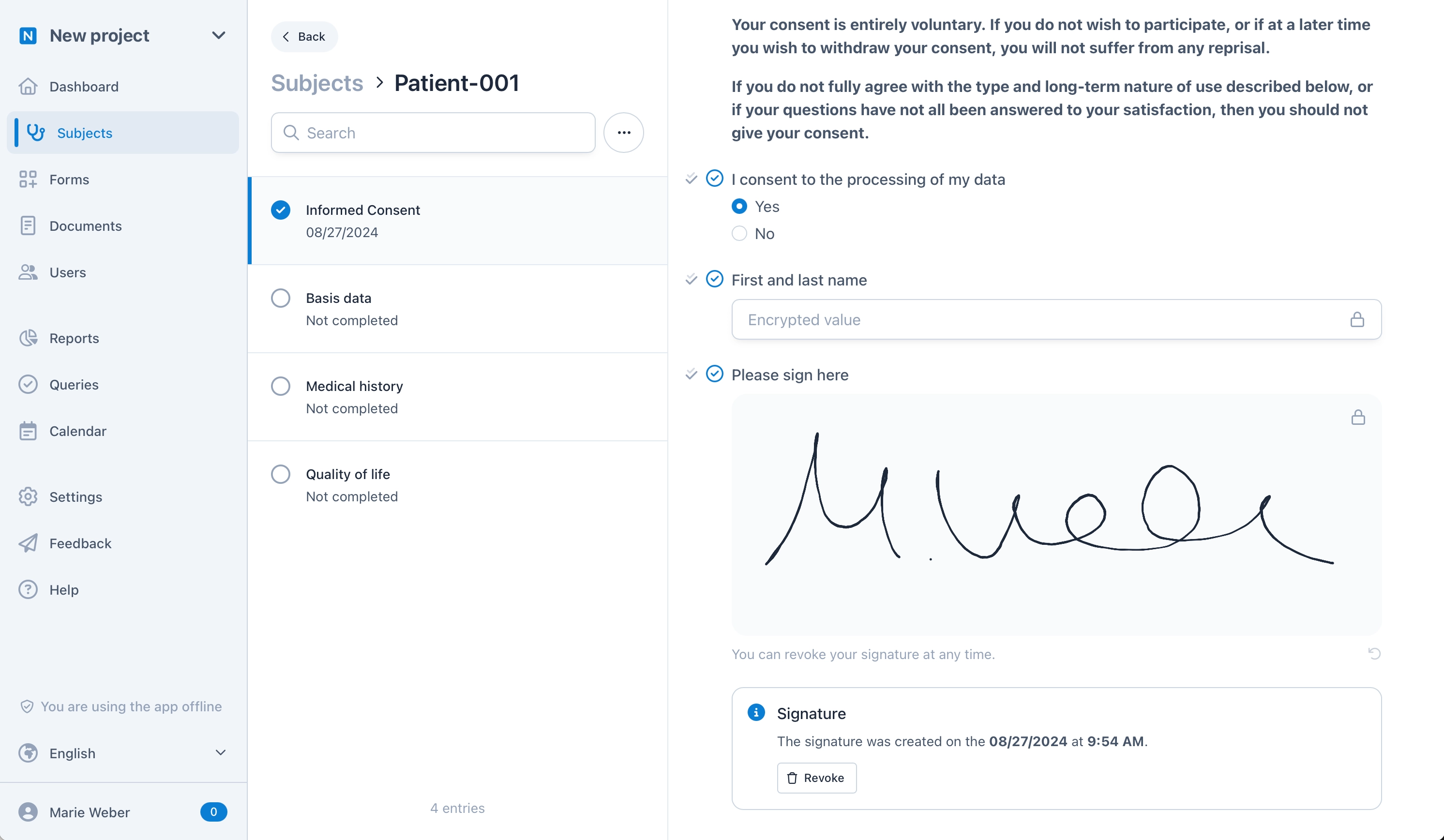
Identifiable Information. Encrypted.
OpenEDC is the first EDC system that offers an end-to-end encryption for personally identifiable information. For example, you can save the name of a patient in an Informed Consent or eConsent form so that only you and the patient can read it – even we have no technical possibility to decrypt and read this data. This also works biometrically via fingerprint, Face-ID or Windows Hello.
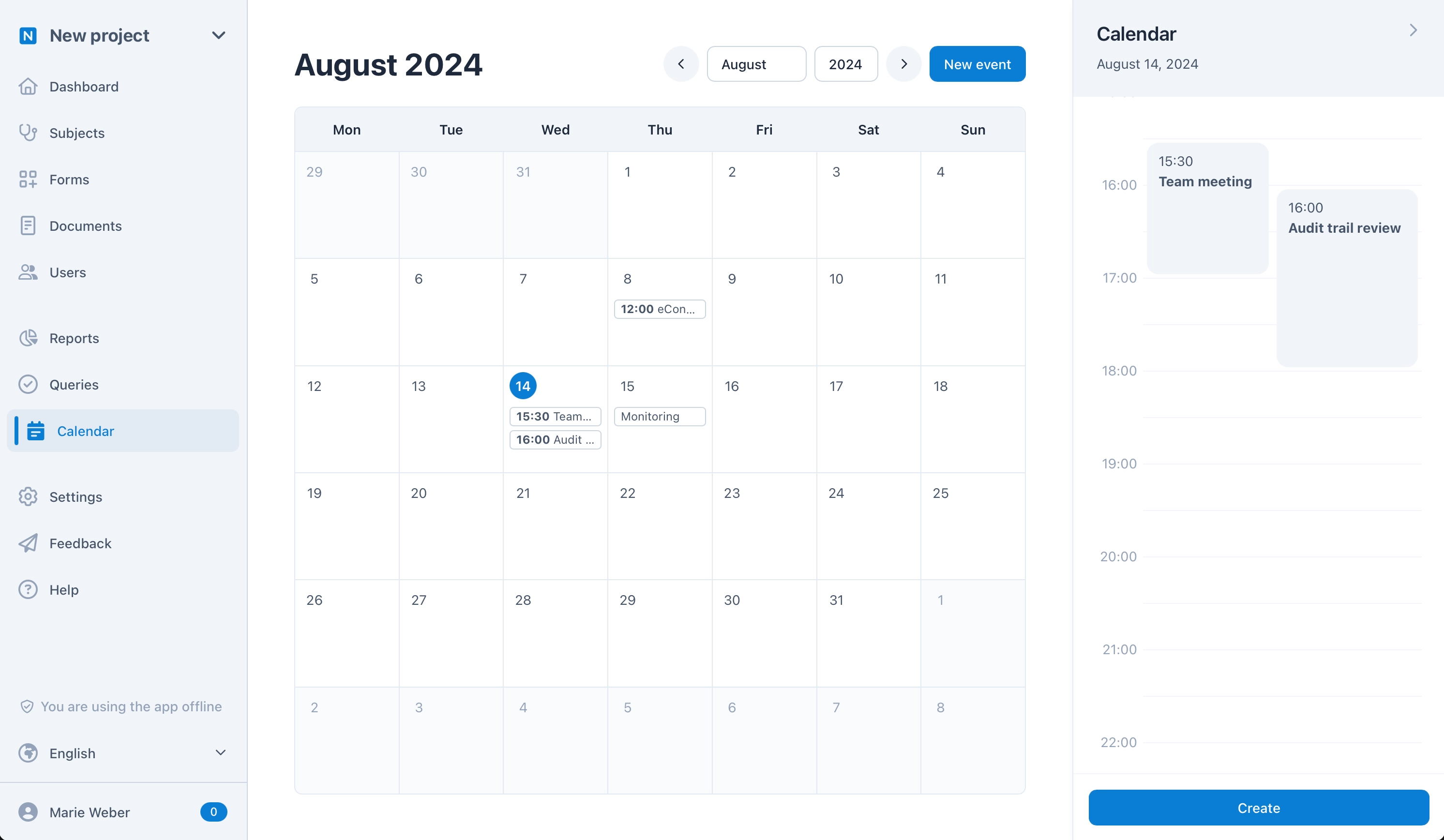
Calendar and Visits
With the calendar module, everyone in your team can create and manage shared or personal dates to keep everyone up to date. In longitudinal studies, the next visits for each subject are automatically displayed in the calendar – this enables central and efficient organization without any manual scheduling.
More Features
OpenEDC offers many features to support you with your study.
-
Pseudonymization
- Manage all subjects of your trial – also completely pseudonymized if required.
-
Randomization
- Different methods for the automatic randomization of subjects.
-
Document Management
- Revision-proof documents for patient consents, SOPs, wikis or similar.
-
Multi-Center Studies
- Creation and management of multiple sites for distributed, multi-center studies.
-
Events and Visits
- Grouping of related forms for longitudinal studies and research projects.
-
Repeating Data
- Patient diaries or medication lists with an arbitrary number of repetitions.
-
Excel and CSV Import
- Existing data can be cleansed, standardized and imported during a guided process.
-
Reason for Change
- Specifying a reason for data changes – in accordance with the GCP regulation.
-
Query Management
- Submit queries on individual data points and monitor their resolution status.
-
Source Data Verification
- Verification of the concordance between collected data and source documents.
-
Electronic Signatures
- Cryptographic electronic signatures after re-login for GCP compliance.
-
Two-Factor Authentication
- Enhanced security through additional authentication using one-time passwords.
References
Practical Experiences
OpenEDC is already being used in several large projects and organizations. These include innovative projects with high demands on modularity and standardization.

“For the EXPERT project funded by the Federal Joint Committee (G-BA), we need a system to collect longitudinal data to evaluate our treatment advice. OpenEDC meets all our requirements and can be used intuitively and accurately by all employees.”

“The major advantages of OpenEDC for us are user-friendliness, extensibility and interoperability. By supporting the CDISC ODM data standard, we can export, import and thus reuse both metadata and clinical data in a widely recognized format.”

“Decentralized data storage and record linkage is a vital factor for us. We use it to implement federated projects without disregarding the needs of the participating sites. Each clinic retains control over its data – and yet comprehensive monitoring is possible with OpenEDC.”
Get in Contact
Would you like to conduct your study simply, securely and cost-effectively? Then get in touch and we will be happy to present OpenEDC to you.
Contact us via email:
mail@openedc.appFeel free to call us:
+49 1590 5368729


