OpenEDC Version 3.0
An integrated notification system, anonymous patient registrations via QR code, and automatic ePRO reminders using push notifications.

Account Manager
We are very excited to officially introduce OpenEDC Health version 3.0 today. This new major release brings three advanced features that make participation in clinical studies even easier and strengthen patient engagement: an integrated notification system with push, email, and in-app notifications, anonymous patient registrations via QR code, and automatic ePRO reminders with support for repeatable events and hourly schedules.
An Integrated Notification System
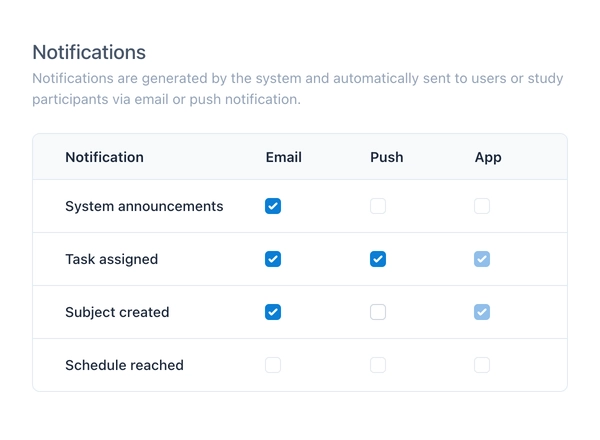
Communication is key to successful clinical trials. With version 3.0, we are introducing a comprehensive notification system that supports push, email, and in-app notifications. The system can be enabled per project and automatically informs users and study participants about important events – whether it’s an upcoming visit, a newly created subject, or an open query.
Push notifications reach participants directly on their smartphone or tablet and sustainably increase compliance. Email notifications ensure that even team members without constant access to the app always stay informed. Unread notifications are clearly displayed in a new slideover that is accessible both from the sidebar and the dashboard.
Anonymous Patient Registrations
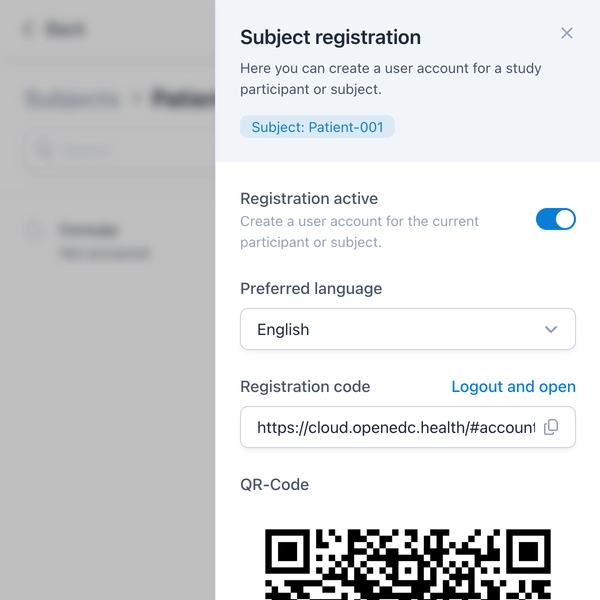
Data protection and privacy are top priorities in clinical research. With version 3.0, study participants or patients can now create anonymous accounts for a study – without providing an email address. This is particularly relevant for sensitive studies or when the anonymity of participants must be guaranteed.
Registration is simple and quick via a QR code provided by the study staff. After selecting a password, participants can install our mobile application on their smartphone or tablet and immediately begin study participation. The app supports many features such as push notifications and offers an intuitive user interface for completing ePROs and questionnaires.
Automatic ePRO Reminders
Regular collection of patient-reported outcomes (ePROs) is essential for many longitudinal studies. Version 3.0 makes this significantly easier through automatic reminders. The system now supports repeatable events with configurable limits as well as hourly schedules – perfect for studies that require daily diary entries or multiple measurements per day.
For example, a pain diary can be configured so that participants receive a push notification every day at 9:00 AM in the morning and 6:00 PM in the evening – for a period of 30 days. It becomes even more flexible through individual schedules per participant. These can either be set by the study staff or, when enabled, adjusted by the participants themselves. This way, study participation can be optimally integrated into everyday life.

Further Updates
Further changes in the last updates briefly summarized:
Limits for Repeatable Visits, Forms, and Sections
For repeatable visits, forms, and sections, a limit for the number of instances can now be configured. This enables more precise planning of study workflows and improves progress calculation. The form status aggregation automatically takes the limit into account and calculates the completion rate of a subject or event based on the total number of repeatable instances.
Repeatable Visits in Schedules
Repeatable visits can now also be mapped in event schedules, with the configured limit being taken into account. Each upcoming visit is displayed with the respective time frame and instance number (e.g., 3. Follow-up) in the subject list and calendar.
Repeatable Visits in Surveys
Surveys via URL or QR code now also support repeatable study events – even without the participant app. This significantly expands the flexibility in data collection and makes it easier to map complex study designs through public surveys as well. The page numbering in surveys automatically takes conditional forms into account and only shows the number of pages that actually need to be answered.
Automatically Hidden Modules
Modules are now automatically hidden in the sidebar when users have neither read nor write permissions for these modules. For granular control, new permissions have been introduced for the modules Forms, Documents, Reports, Queries, Calendar, and Settings. This provides a clearer user interface and improves security through the principle of least privilege.
This concludes this post. Thank you for your interest. If you would like to learn more, please feel free to schedule a non-binding demo.